Translate this page into:
Role of FNAC mapping to guide directed sperm retrieval in infertile males for in-vitro fertilisation (IVF)
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Focal areas of spermatogenesis may exist in men even with small atrophic testis, raised follicle stimulating hormone (FSH) and in men previously considered to be having absence of spermatogenesis such as sertoli cell only syndrome. The study conducted by us has evaluated the regional differences in spermatogenesis by fine needle aspiration cytology (FNAC) mapping according to a standard template. This study aims at finding a possibility of directed sperm retrieval from region with better spermatogenesis for use in assisted reproduction techniques and intra-cytoplasmic sperm injection.
Aimsand Objectives:
The study was conducted with an aim to compare histological grading of spermatogenesis in testicular tissue by FNAC and biopsy in infertile males and to map the pattern of spermatogenesis in the testis of infertile males.
Materials and Methods:
Forty azoospermic men were taken into study and everyone underwent FNAC from eight different segments of one testis. They further underwent open biopsy from the segment, which showed better spermatogenesis. Results of FNAC and biopsy were compared.
Results:
Frequency of normal spermatogenesis was equivalent in all segments of testis by FNAC mapping (P value = 0.986). Sperm detection rate was 90% by eight-segment FNAC mapping in azoospermic participants. Correlation between results of FNAC and biopsy showed 85% concordance and 15% discordance. The eight-segment FNAC mapping showed sensitivity of 100%, specificity of 44.5%, and accuracy rate of 87.5% in detecting normal spermatogenesis.
Conclusion:
It is recommended to do FNAC mapping for every nonobstructive azoospermic subject, before undergoing retrieval procedure for better localization of spermatogenesis.
Keywords
FNAC mapping
sperm retrieval
testis biopsy
INTRODUCTION/BACKGROUND
Male partner is responsible for about half of all infertility cases. Azoospermia is found to be present in 10–15% of men, evaluated for infertility and 1% of general population.[1] Histological diagnosis is required to differentiate between obstructive azoospermia (OA) and nonobstructive azoospermia (NOA).[2] Focal areas of spermatogenesis may exist in men even with small atrophic testis, raised follicle stimulating hormone (FSH) and in men previously considered to be having absence of spermatogenesis such as sertoli cell only (SCO) syndrome.[3] Many studies have documented that even in these cases, one or more seminiferous tubules are involved in spermatogenesis while rest are not. The introduction of intra-cytoplasmic sperm injection (ICSI) in 1992 has led to higher chances of fertilization of ovum and higher pregnancy rates in patients with severely reduced sperm quality.[4] There is a good correlation between the histology found upon diagnostic biopsy and the likelihood of finding mature sperm cells during testicular sperm retrieval and ICSI.[5] Some authors have found a good correlation between fine needle aspiration cytology (FNAC) and open biopsy in men with azoospermia.[6] Obtaining multibiopsy with testicular sperm extraction (TESE) has been reported to cause vascular injury resulting in testicular atrophy and hypogonadism due to excessive tissue removal.[7] The infertile men with azoospermia who are candidates for sperm retrieval and ICSI, fine needle aspiration (FNA) sperm mapping is likely to help in achieving sperm retrievals with reduced potential for damage to the testis from these procedures.[8] Sperm mapping allows for precise, systematic evaluation of all areas of the testis and even the men with the severest forms of infertility, including cancer survivors can become biological fathers.
The study conducted by us has evaluated the correlation between findings of FNAC and open biopsy in correctly assessing the state of spermatogenesis in patients with azoospermia. It also assesses the regional differences in spermatogenesis which was evaluated with FNAC mapping according to template designed and possibility of directed sperm retrieval from region with better spermatogenesis for use in assisted reproduction techniques and ICSI.
STUDY AIMS AND OBJECTIVES
To compare histological grading of spermatogenesis in testicular tissue by FNAC and biopsy in infertile males.
To map spermatogenesis in the testis of infertile males.
MATERIALS AND METHODS
This prospective study on 40 azoospermic participants was conducted in the Department of Surgery and Department of Pathology, Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi from November 2014 to April 2016. The study population constituted infertile males with azoospermia, diagnosed with two samples of semen analysis taken at 2 months interval after sexual abstinence of at least 5 days. They attended male infertility clinic in the Department of Surgery in Lok Nayak Hospital.
Inclusion criteria:
(1) Infertile males of age group 18–60 years.
Exclusion criteria:
Primary or secondary hypogonadism.
Participants with deranged coagulation profile.
Participants with hydrocele, varicocele or any testicular swelling.
Participants with secondary infertility.
Clearance was obtained from institutional ethical clearance committee before conducting the study, and each participant was informed about study in detail in their own language and option was given to participate in study. A well-informed consent was taken from all participants.
Complete history and detailed physical examination were performed for all participants. Semen analysis was performed for all participants with a history of infertility for at least 1 year of cohabitance, at two different occasions 2 months apart after a minimum of 5 days of abstinence. Semen was evaluated for volume, liquefaction time, pH, morphology, sperm counts, and motility according to World Health Organisation (WHO) guidelines 2010. Serum FSH, serum leutinising hormone (LH), and serum Testosterone were estimated for all participants by electro chemiluminescence band immunoassay using a fully automated, closed system. Antisperm antibody was estimated for all participants before and 3 months after testicular open biopsy by sperm immobilization assay. Ultrasound and color Doppler of scrotum was taken to measure testicular size and to rule out any testicular swelling including varicocele.
All participants underwent FNAC of one the testis from eight different segments according to predetermined template, which was made by three horizontal and one vertical imaginary lines dividing testis into eight different segments [Figure 1]. FNAC was performed under spermatic cord block with 1% lignocaine after checking for sensitivity. A template was drawn over testis with methylene blue, and FNAC was performed with a sharp-bevelled, 23-gauge, 1-inch needle using the established suction cutting technique.
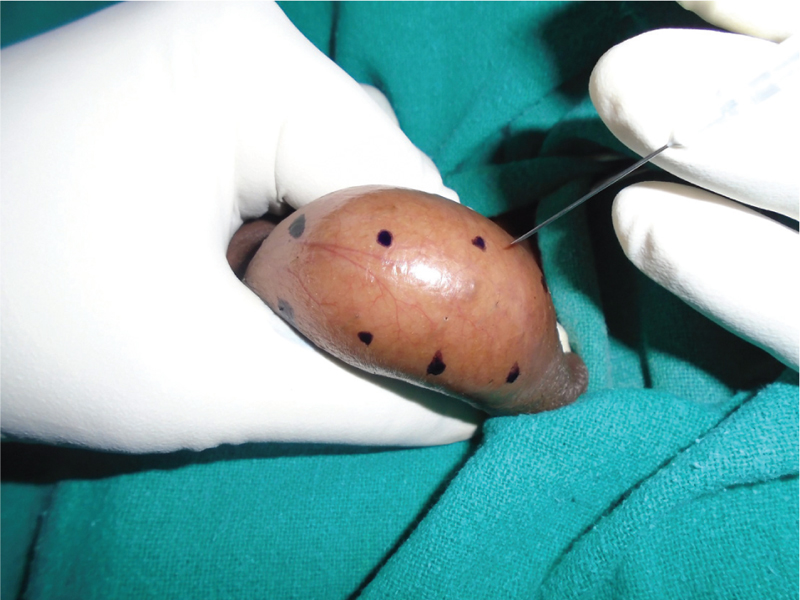
- Template showing eight segments for FNAC mapping on testis
Precise, gentle, in-and-out movements varying from 5 to 8 mm were used to aspirate tissue fragments. Slides were air dried and stained with Giemsa stain and examined under high field microscope for different stages of maturation of germ cells. FNAC grading of spermatogenesis was performed by Chandley’s classification,[9] as normal spermatogenesis, maturation arrest, hypospermatogenesis, and SCO syndrome.
Open biopsy was taken under local anesthesia from one segment which showed a better grade of spermatogenesis on FNAC. An incision was made on tunica albuginea with scalpel blade. On applying gentle pressure on testis the testicular tissue extruded out which was cut with iris scissors. The specimen was transferred into Bouin’s solution. Tunica albuginea was then sutured back with vicryl 4/0 sutures. Biopsy specimen was embedded in paraffin and cut into 6 μm sections. Sections were then stained with hematoxylin–eosin stain and examined under high field microscope, and the results of biopsy were then compared to FNAC findings from the same segment of testis.
The cytological smears and histological sections were examined by different pathologists who were blinded for results from each other.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences version 20.0 software (SPSS Inc., Chicago, IL, United States) and results of FNAC were compared with results of open biopsy in terms of grading of spermatogenesis. Fisher’s tests were conducted for comparative analysis. Statistical significance was defined as P value <0.05.
RESULTS
Analysis of age distribution of infertile male showed that 75% participants belonged to 25–35 years age group. 7.5% participants were <25 years age and 17.5% were 35–45 years age. None of the participants was above 45 years. Mean age for all 40 participants was 31.25 ± 4.65 years (range 22–45 years). Mean duration of infertility among 40 participants was found 7.225 ± 3.38 years (2–15 years).
The histological grading on open biopsy showed normal spermatogenesis in 31 participants while three showed hypospermatogenesis, one showed maturation arrest, and five participants had SCO syndrome.
Each participant had undergone FNAC from eight different segments from one of the testes according to a predetermined template. The results of FNAC were calibrated on the template, and segments were identified which showed normal spermatogenesis [Table 1].
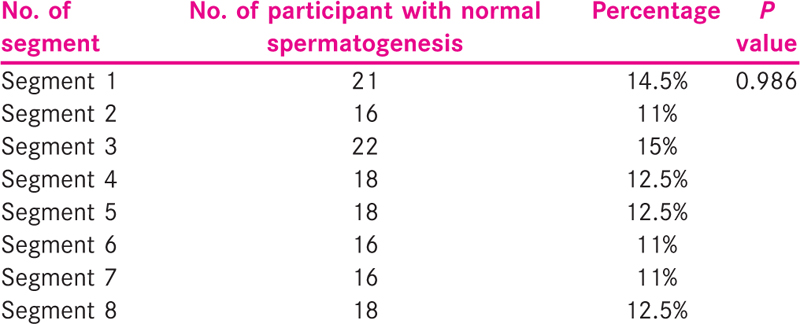
The frequency of normal spermatogenesis in various segments was found to be equivalent without any statistically significant difference among them. In 36 participants out of 40 who underwent FNAC, spermatogenesis was obtained in at least one of the eight segments. Rest of the four participants did not show normal spermatogenesis. There was normal spermatogenesis detected in only one segment in seven participants [Table 2].

According to this data, the number of participants with normal spermatogenesis in five or more segments was 44.5% (16 out of 36), while the number of participants with normal spermatogenesis in four or less segments was 55.5% (20 out of 36), and seven out of 36 (19.5%) participants were having normal spermatogenesis in only one of the eight segments.
A complete concordance between biopsy and FNAC results was found in 34 out of 40 participants (85%) and discordance in six out of 40 participants (15%) [Table 3].

FNAC mapping was found to be 100% sensitive in detecting normal spermatogenesis and 44.5% specific. Positive predictive value was 86%, and negative predictive value was 100%. Accuracy for FNAC mapping was found as 87.5%.
In accordance to the grading of spermatogenesis and comparing the concordance and discordance of FNAC and biopsy among them, Group A included participants with normal spermatogenesis and had 31 participants out of which all 31 were concordant (100%). Group B had three participants with hypospermatogenesis out of which all three (100%) were discordant. Similarly, Group C had one participant with maturation arrest which was discordant (100%), and Group D had five SCO participant who showed three concordant (60%) and two discordant results (40%) [Table 4].

Sperm retrieval was said to be achieved if even one of the eight segments showed normal spermatogenesis. Sperm retrieval rate was 100% in participants with OA (31 out of 31) and 55% (five out of nine) in participants with NOA (P value = 0.007) [Table 5].

Mean value for serum FSH for participants with OA was 6.60 ± 3.80 mIU/ml and for participants with NOA was 10.17 ± 5.55 mIU/ml (P value = 0.171). Mean value for serum LH level in participants with OA was 6.05 ± 2.82 mIU/ml and for participants with NOA 7.16 ± 2.63 mIU/ml (P value = 0.878). Serum Testosterone level for participants with OA was 5.38 ± 3.14 ng/ml and mean for participants with NOA were 4.22 ± 2.05 ng/ml (P value = 0.581). Right testis volume was 11.37 ± 3.77 ml in OA and 10.90 ± 3.20 ml in NOA participants (P value = 0.627), while left testis volume in participants with OA was 11.10 ± 4.62 ml and 9.55 ± 3.53 ml in participants with NOA (P value = 0.244). Antisperm antibodies were measured for all participants before testicular biopsy and 3 months after biopsy. None of the participants was found to have a positive anti sperm antibody (ASA) before as well as after biopsy.
DISCUSSION
History of infertility dates back to the beginning of civilizations, and women had always been held responsible for not being able to give birth to child but in reality, male factors alone contribute to 30–40% of infertility cases and should not be overlooked in the management of infertile couples. Assessment of spermatogenesis is an important component in the diagnostic algorithm of male infertility. Traditionally, open testicular biopsy has been the gold standard in this evaluation, because it provides better information than FNAC. FNAC, on the other hand, is simple, minimally invasive technique that can help in assessing testicular function accurately. Multiple studies have demonstrated convincingly that excellent correlation exists between testicular cytology by FNAC and histology by open biopsy.[10,11,12] With advent of assisted reproduction techniques in last two decades and the development of technique like ICSI, it has now become possible to achieve pregnancy even with a single spermatozoa. ICSI also allowed spermatozoa with limited intrinsic fertilizing capacity to reliably fertilize eggs, including immature spermatozoa derived from male reproductive tract.[4] Open conventional TESE is performed using single or multiple small incisions in the tunica albuginea.[6,13] Multibiopsy TESE increased the chances of sperm retrieval but it also significantly raised chances of vascular injury resulting in testicular atrophy and hypogonadism due to excessive tissue removal.[7,14] This led to the necessity to develop testis-sparing technique for retrieval of spermatozoa. Schlegel developed a technique of microdissection TESE, which involved a single large, 3–4 cm equatorial or polar incision in the testicular tunica albuginea, followed by physically inverting the testis to expose the entire parenchyma for inspection and subsequently using operative microscopy to harvest dilated and opaque seminiferous tubules selectively.[15,16] Mapping of testis with FNAC to know the location of spermatozoa and then directed sperm retrieval procedure, was considered a better alternative by Turek et al. for retrieval of spermatozoa for ICSI.[17] This findings paved the way for the development of a relatively safe, templated, multisample FNA procedure for sperm detection in azoospermic participants. In NOA, it is very difficult to detect and retrieve spermatozoa mainly due to patchy and focal nature of spermatogenesis in participants with testicular failure.[18,19] In our study also it was found that the nature of spermatogenesis was focal in testis of men of both obstructive as well as NOA. Number of participants with normal spermatogenesis in five or more segments was 44.5%, while number of participants with normal spermatogenesis in four or less segments was 55.5% and seven out of 36 (19.5%) participants were having normal spermatogenesis in only one of the eight segments. Also only two participants had normal spermatogenesis in all eight segments. So only 5.5% participants had 100% chance of sperm retrieval irrespective of the fact that from where retrieval isperformed. Rest all participants had benefited from performing a template based retrieval with prior FNAC. Sperm could be retrieved in almost 100% cases of OA but only in 50% cases of NOA.[20] We were able to document normal spermatogenesis in 36 out of 40 participants in at least one of the segments (90%). Four out of 40 participants had not shown normal spermatogenesis in any of the mapping segment. In seven participants, normal spermatogenesis was found in only one out of eight segments. In 20 out of 36 participants (55.5%) normal spermatogenesis was present for ≤4 FNAC mapping segments, while remaining 16 out of 36 participants (44.5%) had normal spermatogenesis in ≥5 FNAC mapping segments which shows importance of pre-op FNAC mapping in confirming spermatogenesis. Sperm detection rate for our study was 90%. Similar studies points toward increasing sperm detection rate with increasing number of mapping sites. Weiss et al. reported a 35.7% sperm detection by using three FNAC sites per testis[21] and Turek et al. found detection rate as 47% for 7.6 mapping sites per testicle.[19] In our study, all segments showed equivalent chances of showing normal spermatogenesis. Normal spermatogenesis was found in 31 out of 36 biopsies in participants with at least one segment of normal spermatogenesis, hence concordance rate from FNAC to open biopsy was 86%. Remaining five out of 36 participants had a discordant result in biopsy (14%). In these five participants biopsy was not able to retrieve spermatozoa even though FNAC had shown its presence. In remaining four participants who were unable to show normal spermatogenesis in any of the eight segments, biopsy was performed and found concordant to results of FNAC in three out of four cases (75%). Discordant result was seen in one participant (25%). Our findings on correlation is similar to those of other researchers, Gottschalk-Sabag et al., found a correlation rate of 87% in a study conducted with 54 testes.[10] Tournaye et al. found 94% correlation between FNAC and biopsy.[22] In our study, FNAC mapping with eight aspiration sites/testes was found 100% sensitive and 44.5% specific in detecting normal spermatogenesis in azoospermic participants with positive predictive value of 86% and negative predictive value of 100% and an accuracy rate of 87.5%. According to Silber et al. only histopathology has been shown to predict the probability of finding the sperm with sensitivity of 86% and specificity of 93%, and accuracy of 0.83 in SCO participants and 0.55 in maturation arrest participants.[23] Serum FSH, LH and testosterone was measured for all participants in our study and two out of 40 participants showed serum FSH value of two times the normal limit. In those two participants, one man had shown normal spermatogenesis in only one segment of mapping pointing toward only foci with normal spermatogenesis and follow-up biopsy from same segment led to retrieval of sperm. In another subject, none of the segments showed normal spermatogenesis and biopsy provided the grading of SCO. So our study points out the prevalence of focal spermatogenesis and the importance of knowing areas with good spermatogenesis beforehand on performing sperm retrieval. A better and more individualistic approach would be performing FNAC and simultaneous examining slides for spermatogenesis one by one and once a segment is found with normal spermatogenesis, sperm retrieval to be performed. In this way, the invasiveness of doing all eight segments mapping can be reduced, and similar results may be obtained. No segment is more likely to have normal spermatogenesis than any other segment; hence, a one-by-one approach according to template can be used. Clearly, with the help of ICSI, mapping can help in treatment of infertility of men even with most severe forms of testicular failure.
CONCLUSION
To conclude, eight segments FNAC showed equal frequency of normal spermatogenesis in all segments of testis and 100% sensitivity with 44.5% specificity in detecting normal spermatogenesis. Based on our findings, we recommend FNAC mapping for every azoospermic subject, before undergoing retrieval procedure, which can be performed on a one-by-one approach by evaluating FNAC slides simultaneously after taking them and performing retrieval once an area with normal spermatogenesis is identified. In this way, a more individualistic approach can be beneficial in reducing unnecessary eight segments FNAC.
Consent
A well-informed, written consent was obtained from all participants before enrolling them for study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Relationship between classic histological pattern and sperm findings on fine needle aspiration map in infertile men. Hum Reprod. 2000;15:1973-7.
- [Google Scholar]
- Simplified recovery, preparation and cryopreservation of testicular spermatozoa. Hum Reprod. 1995;10:1623-6.
- [Google Scholar]
- Pregnancies after intra-cytoplasmic sperm injection of single spermatozoa into an oocyte. Lancet. 1992;340:17-8.
- [Google Scholar]
- Predictors of sperm recovery and azoospermia relapse in men with nonobstructive azoospermia after varicocele repair. J Urol. 2012;187:222-6.
- [Google Scholar]
- Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997;49:435-40.
- [Google Scholar]
- Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688-92.
- [Google Scholar]
- The value of testicular ’mapping’ in men with non-obstructive azoospermia. Asian J Androl. 2011;13:225-30.
- [Google Scholar]
- Cytogenetics and infertility in man II. Testicular histology and meiosis: Results of a five year study of men attending a subfertility clinic. Ann Hum Gen. 1976;40:165-76.
- [Google Scholar]
- Fine needle aspiration of the testis and correlation with testicular open biopsy. Acta Cytol. 1993;37:67-72.
- [Google Scholar]
- Assessment of testicular cytology by fine needle aspiration as a diagnostic parameter in the evaluation of the azoospermic subject. Fertile Steril. 1992;57:858-65.
- [Google Scholar]
- Testicular fine-needle aspiration in infertile men: Correlation of cytologic pattern with biopsy histology. Am J Surg Pathol. 2001;25:71-9.
- [Google Scholar]
- Presence of mature sperm in testicular parenchyma of men with nonobstructive azoospermia: Prevalence and predictive factors. Urology. 1997;49:91-5.
- [Google Scholar]
- Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190-4.
- [Google Scholar]
- Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131-5.
- [Google Scholar]
- Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168:1063-7.
- [Google Scholar]
- Systematic fine-needle spiration of the testis: Correlation to biopsy and results of organ mapping for mature sperm in azoospermic men. Urology. 1997;49:743-8.
- [Google Scholar]
- Testicular biopsy in the study of male infertility: Its current usefulness, histologic techniques, and prospects for the future. Hum Pathol. 1979;10:569-84.
- [Google Scholar]
- Diagnostic findings from testis fine needle aspiration mapping in obstructed and nonobstructed azoospermic men. J Urol. 2000;163:1709-16.
- [Google Scholar]
- Surgical sperm retrieval for intracytoplasmic sperm injection. Int J Androl. 1997;20(Suppl 3):69-73.
- [Google Scholar]
- [Seminiferous tubule cytological pattern in infertile, azoospermic men in diagnosis and therapy] Harefuah. 1997;132:614-8. 680
- [Google Scholar]
- Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod. 1997;12:80-6.
- [Google Scholar]
- Distribution of spermatogenesis in the testicles of azoospermic men: The presence or absence of spermatids in the testis of men with germinal failure. Hum Reprod. 1997;12:2422-8.
- [Google Scholar]







