Translate this page into:
A pilot study on effect of autologous platelet-rich plasma on refractory thin endometrium in frozen embryo transfer cycle
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
During assisted reproductive technology (ART) treatments, one of the most common reasons for cancellation of the cycle is thin endometrium. Refractory endometrium which doesn’t respond to standard medical therapies can be the aetiology of recurrent implantation failure. Thin endometrium remains an unsolved problem in the treatment of infertile women. Autologous PRP is known to help tissue regeneration.
Aims:
To evaluate the effect of intrauterine infusion of platelet-rich plasma in case of thin endometrium and its impact on endometrial thickness and clinical pregnancy rate.
Materials and Methods:
Our study was prospective randomised controlled trial done from 1st October 2018 to 1st April 2019 at Reproductive Biology & IVF Centre, Maulana Azad Medical College, New Delhi, India. Twenty- four women undergoing frozen embryo transfer with history of thin endometrium (less than 7 mm) with normal hysteroscopic examination were enrolled. Patients with uncorrected asherman syndrome, submucosal polyp, fibroid or congenital uterine anomaly were excluded from study. From day 2 of menses Tab estradiol valerate (tablet Estrabet, Abott India) was started in a dose of 6–8 mg/day. It was increased up to 12 mg/day gradually after reviewing endometrial thickness serially. Patients with thin endometrium on day 10/11 received PRP on day 11 and repeat dose after 48 hours if endometrial thickness was less than 7 mm. Frozen embryo transfer was done in patients who achieved endometrial thickness 7 mm or more.
Results:
The mean pre-treatment endometrial thickness was 4.68±0.96 mm, which significantly increased to 6.65±0.52 mm, post treatment (P < 0.05). Four patients out of 24 in our study could not achieve an optimal pattern of endometrium after treatment and embryo transfer was postponed. The positive beta human chorionic gonadotropin rate was 55%, and clinical pregnancy rate was 45% among them.
Conclusion:
PRP was found to play an effective role in thin endometrium.
Keywords
Assisted reproduction technology
frozen embryo transfer
platelet rich plasma
thin endometrium
INTRODUCTION
Prediction of pregnancy outcome following in vitro fertilization(IVF) remains elusive. The endometrium plays a major role during assisted reproductive technology (ART) treatment. Implantation involves a synchronised process of attachment and invasion of the embryo into the endometrium. It starts with the apposition of the maternal and embryonic epithelium, followed by adhesion, penetration and invasion of placental cells deep into the endometrium.[1] The tissue of the endometrium contains various receptors of growth factors, cytokines, and lipids which are vital for endometrial and embryonic development which is necessary for successful implantation.[2] In frozen embryo transfer (FET) cycles, implantation mainly depends on endometrium receptivity and embryo quality and technique of transfer. On transvaginal ultrasonography endometrium thickness, blood flow and its pattern can be measured. A thin endometrium is encountered in 2.4% of ART cycles.[3] Though pregnancies have been reported at 4 and 5 mm it is apparent that an endometrial thickness <7 mm is associated with a trend toward lower probability of pregnancy.[4] There are around 0.6–0.8% cycles of IVF in which embryo transfer is cancelled because of thin endometrium, that is less than 7 mm.[5] Angiogenesis is an important parameter for endometrial growth. Vascular epidermal growth factor (VGEF) is a significant mediator for hormonal dependent endometrium growth. The decreased expression of VGEF receptor due to increased resistance in the uterine arteries results in poor endometrial growth. Hormonal manipulation like an extended dose of estrogen or improving endometrial perfusion by low dose aspirin, pentoxifylline and vitamin E, sildenafil, and new modalities like Granulocyte colony-stimulating factor (G-CSF) are used for endometrial expansion.[6] However, these methods cannot improve all cases with thin endometrium.
Platelet- rich Plasma (PRP) application is a successful catalyst in the healing process for wide varieties of conditions. PRP therapy began gaining popularity in the mid 90s.[7] Methods of harvesting and using PRP are becoming widely practised in the fields of sports medicine, dentistry, dermatology and cosmetology and aesthetic medicine. Main function is haemostasis by attaching to any breach in vessel where platelets aggregate, activate and release granules. Alpha granules contain clotting mediators such as factor V, factor VIII, fibrinogen, fibronectin, platelet-derived growth factor (PDGF) and chemotactic factors.[8] Activated platelets release granules by exocytosis which contain more than sixty different biologically active substances that are involved in processes of tissue regeneration, angiogenesis, epithelialization, and proliferation. PRP infusion is newer modality of treatment in thin endometrium. It is prepared from autologous blood plasma that is concentrated with platelets.
The aim of our study was to evaluate the effect of intrauterine infusion of platelet-rich plasma in case of thin endometrium and its impact on endometrial thickness and clinical pregnancy rate.
MATERIAL AND METHODS
Study design: Prospective clinical intervention
Study setting: Reproductive Biology & IVF Centre, Maulana Azad Medical College (MAMC), New Delhi
Study duration: 6 months (1st October 2018 to 1st April 2019)
Inclusion criteria:
Women aged above 25 years.
Previously cancelled at least one cycle because of thin unresponsive endometrium.
Exclusion criteria:
Patient on any anticoagulant therapy.
Patients with uncorrected Asherman’s syndrome, submucosal polyp or fibroid and congenital uterine anomaly.
Patient with recent history of fever or viral illness.
Patient with history of systemic use of corticosteroid in last 2 weeks.
Patient with poor quality of embryos.
After considering the inclusion and exclusion criteria, we recruited 24 women with thin endometrium for PRP instillation. The study protocol was approved by the Ethics committee of MAMC. Subjects were recruited from fertility clinic of the hospital. All of the participants were provided with informed consents after they were counselled for infertility treatments and routine IVF procedures.
All baseline investigations as CBC, blood group, coagulation profile, renal function test, thyroid profile, serum prolactin, HIV, HbsAg, anti HCVAb and VDRL were done when patient enrolled in the study. Baseline transvaginal ultrasound was done on day 2/3 of menses to check endometrial thickness (ET) and rule out ovarian cyst. Oral contraceptive pills were given for 21 days to the patients who had ovarian cyst of size more than 10 mm and again reviewed on day 2 of the next cycle. From day 2/3 of menses, Tab estradiol valerate (tablet Estrabet, Abott India) was started in dose of 6–8 mg/day. It was gradually increased up to 12 mg/day in divided doses after reviewing endometrial thickness serially. After a longitudinal view of the uterus was obtained, the thickness of the endometrium was measured at the maximal distance between each myometrial/endometrial interface using vaginal ultrasound by the same operator to minimize the subjective error. Using Power Doppler, vascular pattern was noted. When blood flow was present in the hypoechoic endometrio–myometrial junction, it was graded as zone 1 vascularity. When it was up to the outer hyperechoic line of the endometrium, it was zone 2 vascularity. When it was in the intervening hypoechoic area, it was known as zone 3 vascularity and when blood flow reached the central echogenic line, it was known as zone 4 vascularity. After informed consent, patients who have endometrial thickness less than 7 mm on day 10 were subjected to intrauterine infusion of autologous PRP on day 10/11. Second dose of PRP was given to those patients who had endometrial thickness less than 7 mm after 48 hrs of the first dose. Frozen embryo transfer was done in women who achieved endometrial thickness of at least more than 6 mm with zone 3/4 vascularity after 48h of last dose of PRP. Luteal support was given with injectable and intravaginal progesterone to all women who underwent embryo transfer. After 15 days of embryo transfer, pregnancy was confirmed with serum bhcg level> 50ng/ml. Biochemical pregnancy was defined when bhcg level> 50ng/ml while clinical pregnancy was defined when cardiac activity was seen four weeks after the embryo transfer.
PRP preparation technique
Take one 15 ml tube. 1.5 anticoagulant (PRP Max, Cryo Cell Pvt Ltd) is added to the tube and then 13.5ml blood was collected using a scalp vein set at 18 No. directly to the anticoagulant added tube. Ratio of 1.5ml anticoagulant to 13.5 ml blood should be maintained.
Caps are tightly closed and mixed well by inversion several times.
Centrifuge for 15min at 1500 rpm.
Plasma rich in platelets will be separated. At least 50% volume should be plasma on top and there should not be any buffy coat on the RBC layer. If plasma is less, centrifugation needs to be increased and if buffy coat forms, centrifugation needs to be reduced.
The supernatant is taken in another tube and again centrifuged.
Centrifuge at 3000 RPM for 15 min to get platelet pellet. Upper plasma layer should be very clear and should not show any swirling platelets when shaken gently. If swirling, seen centrifuge further 10min to get clear plasma.
Leave behind 10% plasma above the pellet and discard the remaining supernatant using sterile pipette.
Re- suspend the platelet pellet in the remaining plasma using sterile pipette, load the intrauterine catheter and used immediately.
STATISTICAL METHODS
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0. The comparison of continuous variables between the groups was performed using Student’s t test. Nominal categorical data between the groups were compared using Chi-squared test. For all statistical tests, a P-value less than 0.05 will be taken to indicate a significant difference.
RESULTS
In the study, 24 patients were enrolled who fulfilled the inclusion and exclusion criteria. Optimal endometrial thickness could not be achieved in four patients and hence were excluded.
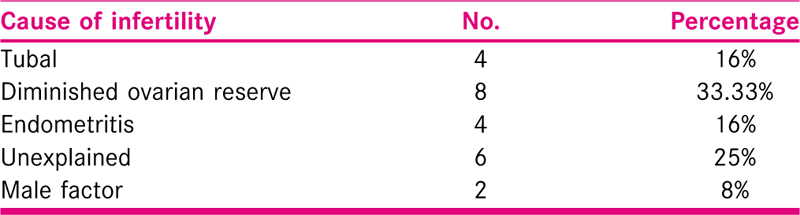
The average age of the patients was 39.04 years. The mean duration of infertility in these women was 19.02 years. Out of which, 14 women had primary infertility while 10 had secondary infertility. Various causes of infertility among these women included tubal (n=4), endometritis (n = 4), unexplained (n = 6), DOR (n = 8) and male factor (n = 2) [Table 1].
Patients were given estradiol valerate (tablet Estrabet, Abott India) from day 2/3 of menses. The mean duration of estrogen given was 18.37 days. The mean of pre-treatment endometrial thickness was 4.68±0.96 mm while after treatment with PRP it was 6.65±0.52 mm (P < 0.05) [Figure 1]. The mean of pre-treatment flow zone is 1.96± 0.75 while that of post-treatment is 3.63±0.49 (P < 0.001) [Figure 2]. Biochemical pregnancy was seen in 55%, while clinical pregnancy rate was 45% [Table 2].
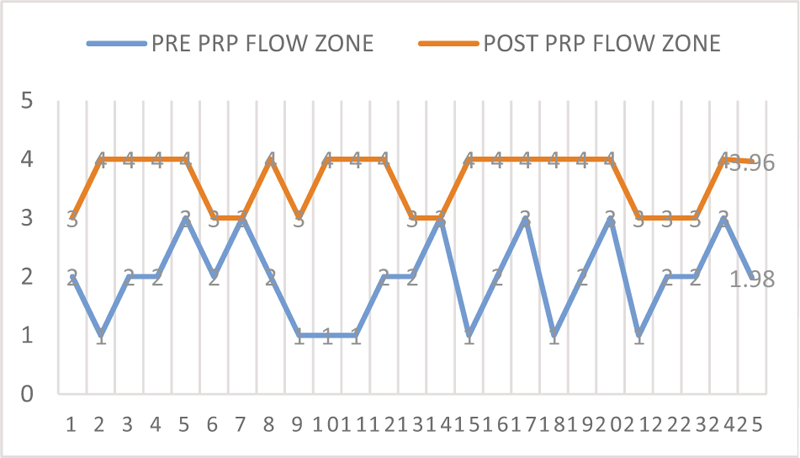
- Post and pre PRP flow zone of the endometrium.
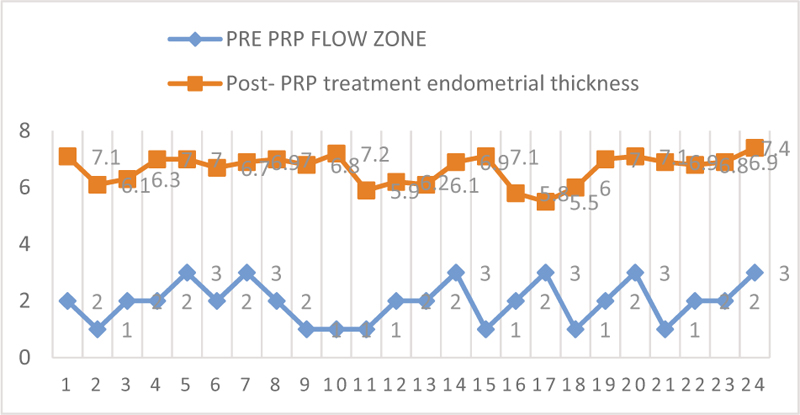
- Endometrial thickness pre and post PRP treatment.

DISCUSSION
Optimal endometrium thickness is one of the critical factors for successful embryo implantation. It is accepted that an endometrial thickness of less than 7 mm would be considered as refractory and that would entail to altered pregnancy and live birth. Refractory endometrium can be idiopathic, congenital, surgical (uterine curettage); caused by inflammatory processes, infections and radiation therapy, among other causes.[9] Consequently, endometrium preparation has been considered a crucial step for embryo transfer. PRP is an autologous blood plasma that has been enriched with platelets. It stimulates proliferation and regeneration with a large amount of growth factors and cytokines, including PDGF, TGF, VGEF, EGF, fibroblast growth factor (FGF), insulin-like growth factor I, II (IGF I, II), interleukin 8 (IL-8) and connective tissue growth factor (CTGF).[10] PRP has a role in improving implantation rate and clinical pregnancy rate with thin endometrium patients.[11] Tandulwadkar et al. assessed not only the endometrial thickness, but also the endometrial vascularity after PRP infusion. They included 68 patients in their study, 64 achieved optimal thickness for FET cycle. Similar to our study, of the 64 patients that underwent FET, endometrial vascularity increased in all of them. Average mean lining thickness before PRP infusion was 5mm, and 7.22 mm after the infusion. They also stated that it decreases the cycle cancellation and psychological and financial stress of infertile women.[12] The main advantage of autologous PRP is that it is safe, with no side effects, cheap, easily accessible treatment of refractory endometrium.
In 2015, Chang et al.[13] studied the intrauterine infusion of PRP for thin endometrium. They instilled PRP prepared from patient’s own blood in 5 women with <7mm endometrial thickness. Post treatment the ET was increased >7mm. All these patients became pregnant after successful embryo transfer, only 1 had missed abortion. Chang Y et al.[14] did a prospective cohort study and included 42 in study group and 30 patients in control. After PRP infusion, the average endometrium thickness on the day of progesterone administration in PRP group was 7.65 + 0.22mm which was significantly thicker than the control group (6.52 +0.31). They concluded that PRP plays an active role in promoting endometrium proliferation, improving embryo implantation rate and clinical pregnancy rate. These results agree with our study. In the same way, 10 patients were evaluated by Zadehmodarres et al who had history of cancelled cycles due to inadequate endometrial growth (<7mm). They also instilled PRP twice, and found that the endometrial thickness increased to more than 7 mm. Five patients became pregnant (50%) after the embryo transfer. They concluded that PRP was effective for endometrial growth in infertile women with a thin endometrium.[11]
Garcia-Velasco et al performed an extensive review on the management of refractory endometrium. The review included studies with estradiol valerate, as well as various drugs including acetylsalicylic acid (aspirin), sildenafil, vitamin E, GnRH agonists, HCG, l-arginine, pentoxifylline among others; as well as autologous preparations as growth factors, mainly G-CSF, stem cells, PRP and bone marrow. They concluded, that despite the vast basket of resources available, it is not easy to provide an evidence-based approach on how to improve refractory endometrium.[15]
CONCLUSION
Thin refractory endometrium still remains one of the challenges. The use of the PRP preparation and its effect on the endometrium seems to offer benefits to refractory endometrium, providing an increase in endometrial receptivity and a consequent increase in implantation rates. Being an autologous resource, it is harmless to the patient, easy to obtain and of very low cost. It is recommended to be included in the different protocols for endometrial preparation in assisted reproduction techniques. Further studies are recommended in terms of the population size as well as the approach of comparative studies between drugs and autologous preparations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Principles and practice of assisted reproductive technology (second). The Health Science Publisher; 2018. p. :103.
- Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol. 2001;45:597-605.
- [Google Scholar]
- Endometrial thickness and pregnancy rates after IVF: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:530-41.
- [Google Scholar]
- The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reproductive Biology and Endocrinology. 2012;10:100.
- [Google Scholar]
- The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37.
- [Google Scholar]
- Treating patients with “thin” endometrium-an ongoing challenge. Gynecological Endocrinology. 2014;30:409-14.
- [Google Scholar]
- Platelet-rich plasma therapy: another appealing technology for regenerative medicine? Future Medicine 2016:355-7.
- [Google Scholar]
- Platelet α-granules: basic biology and clinical correlates. Blood Reviews. 2009;23:177-89.
- [Google Scholar]
- Defective endometrial receptivity. Fertil Steril. 2012;97:1028-32. doi: 10.1016/j.fertnstert.2012.03.039
- [Google Scholar]
- Platelet rich plasma: a short overview of certain bioactive components. Open Medicine. 2016;11:242-7.
- [Google Scholar]
- Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assisted Reproduction. 2017;21:54.
- [Google Scholar]
- Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: a pilot study. Journal of Human Reproductive Sciences. 2017;10:208.
- [Google Scholar]
- Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. International Journal of Clinical and Experimental Medicine. 2015;8:1286.
- [Google Scholar]
- Platelet-rich plasma administration has benefit for infertile women with thin endometrium in frozen blastocyst-stage embryos transfer program. Fertility and Sterility. 2017;108:e77.
- [Google Scholar]
- Strategies to manage refractory endometrium: state of the art in 2016. Reprod Biomed Online. 2016;32:474-489. doi: 10.1016/j.rbmo.2016.02.001
- [Google Scholar]







