Translate this page into:
Management of Polycystic Ovary Syndrome in India
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
INTRODUCTION
Polycystic Ovary Syndrome (PCOS) is one of the most common endocrinopathy affecting women.[1] It has an unknown etiology and is recognized as a heterogeneous disorder that results in overproduction of androgens, primarily from the ovary, and is associated with insulin resistance (IR).[1] The Rotterdam 2003 criteria defines PCOS as incidence of any two of the three key criteria, namely, oligoovulation and/or anovulation, excess androgen activity and polycystic ovaries(PCO).[1,2] However, the terminology used in the context of PCOS needs to be revisited to reflect the actual clinical nature of PCOS.
Studies of PCOS in India carried out in convenience samples reported a prevalence of 3.7% to 22.5%,[3,4] with 9.13% to 36% prevalence in adolescents only.[5,6] The wide variation in prevalence might be due to heterogeneous presentation of symptoms, diagnostic criteria practiced, limitations in diagnosis, age groups, and ethnic populations studied. Therefore, it is essential to consider these factors before diagnosis and/or management is initiated. Further studies are needed to understand the dynamics in prevalence rates of PCOS across India. Although there is a paucity of data from large scale surveillance studies, the higher incidence of PCOS risk factors (high body mass index (BMI), IR) in India, suggests that the real extent of the problem might be currently underestimated.[7]
The most common symptoms of PCOS can range from menstrual disorders, infertility, hyperandrogenemia to metabolic syndrome (MS).[8] Elevated insulin levels due to IR may lead to development of PCOS by contributing to the underlying abnormalities seen in the hypothalamic-pituitary-ovarian axis. The resulting complex of physiological dysfunction produced by interrelated metabolic and hormonal factors, predisposes patients with PCOS to different complications like endometrial hyperplasia and cancer, cardiovascular disease (CVD), miscarriage, and acanthosis nigricans (AN).[8] The complications add to the burden faced by patients, besides effecting social and emotional wellbeing, especially in adolescents, who are under the impression of being afflicted by a 'disease'.
Efficient management of PCOS provides a prospective window of opportunity to avoid the risk of associated complications. Treatment is broadly aimed at tackling (IR), effects of hyperandrogenism, irregular menstruation, and infertility. However, given the complex nature of PCOS, tailoring treatment options to the needs of individual patients can be a difficult clinical exercise. Long-term risks of PCOS must be balanced against current acute needs of the patients like the desire for continued fertility and the need to ameliorate the cosmetic challenges associated with PCOS. Due to its heterogeneous nature, effective management of PCOS needs a sustained, multi-pronged strategy with inter-disciplinary expertise, based on strong evidentiary framework to guide the standardization of care. However, in contemporary clinical practice in India, successful interdisciplinary cross-linking of efforts is stymied by the lack of awareness about PCOS and guidelines addressing its management. Further, in view of the higher risk of PCOS in Indian women, and the relative lack of medical infrastructure to deal with the chronic outcomes of PCOS, effective, evidence-based treatment guidelines for India are an immediate necessity. The current good clinical practice recommendations (GCPR) are an effort to provide a comprehensive framework for addressing issues relating to the management of PCOS in India. It is aimed at providing scientific evidence and well-balanced information on multi-disciplinary approach for the management of PCOS to health care providers in India.
METHODOLOGY
Systematic review of literature was conducted to provide the best possible evidence base for the GCPR. Existing guidelines, meta-analyses, systematic reviews, key cited articles relating to PCOS were reviewed by a group of doctors and recommendations relevant to Indian scenario were framed. The recommendations were discussed by an expert panel of gynecologists, physicians, ultrasonologists, endocrinologists, dematologists, and pediatricians in a series of meetings. GCPR for each section of the guidelines were discussed and where there was little or no evidence, the panel relied on experience, judgment and consensus to make their recommendations. The current consensus GCPR are developed in accordance to the American Association of Clinical Endocrinologists (AACE) protocol for standardized production of clinical practice guidelines. Recommendations are based on clinical importance (graded as A: strongly recommended, B: suggested, and C: unresolved) coupled by four intuitive levels of evidence (1 = 'at least one randomized controlled trial (RCT) or meta-analysis of RCTs', 2 = 'at least one non-randomized or non-controlled, prospective epidemiological study', 3 = 'cross-sectional or observational or surveillance or pilot study' and 4 = 'existing guideline or consensus expert opinion on extensive patient experience or review').[9]
RISK FACTORS FOR THE ASSESSMENT OF PCOS
Current Evidence:
Several risk factors are associated with the incidence of PCOS, but not all are causative or predisposing factors for PCOS. However, their presentation is indicative of the incidence of PCOS. Thus the risk factors included in the current section indicate the risk for PCOS diagnosis but not the etiological likelihood of disease development. A preliminary understanding of the clinical characteristics and medical history of the patients is an invaluable resource for assessing the risk of PCOS incidence.
Biochemical risk factors
BMI is a key risk factor associated with the incidence of PCOS (mean BMI: 29.3 ± 7.5 vs. 25.6 ± 5.8 kg/m2, p < 0.001 in women with and without PCOS);[10,11] higher BMI has been implicated as an important indicative marker of PCOS status. In women with PCOS, changes in BMI during adolescence are positively associated with changes in waist circumference (p < 0.0001), low density lipoprotein-cholesterol (LDL-C) (p = 0.01), triglycerides (TG) (p = 0.008), and systolic blood pressure (SBP) (p = 0.002).[12] In adults, a BMI ≥ 23 kg/m2 is considered overweight /obese,[13] whereas in adolescents BMI > 97.5 th percentile for age and gender are regarded as overweight/obese.[14,15] In addition, development of clinical features of PCOS is often preceded by a history of weight gain,[16,17] and factors independently associated with BMI: higher energy intake and glycaemic index, low physical activity, smoking, alcohol intake.[18]02
Development of IR and dysregulation of lipid metabolism are seen even in the early stages of PCOS.[19] Significantly higher IR (fasting serum insulin) is observed in patients with PCOS with apparently normal oral glucose tolerance test (OGTT).[20] Similarly, early signs of lipid metabolic dysregulation (elevated serum total cholesterol, TG and LDL-C levels and carotid intima-media thickness) were observed in age-matched patients with PCOS between 18-35 years.[19] Presence of family history of PCOS or diabetes or an inadequate lifestyle have also been shown to be important risk factors for incidence of PCOS [Figure 1].[21,22]
- Modified Ferriman-Gallwey hirsutism scoring system
Clinical risk factors
Patients with normal menstrual cycle compared to patients with oligo/amenorrhea show significantly better metabolic parameters (BMI, fasting insulin, and Homeostasis Model Assessment- insulin resistance (HOMA-IR).[23] Due consideration to the patient's demographic profile is important in determining the PCOS status of a patient, since the risk and presentation varies in different patient groups. In adolescent patients, the diagnostic criteria of PCOS based on the signs and symptoms often overlap with the characteristics of normal puberty. In adolescents and younger women with PCOS, primary risk factors include disturbances in periodicity/timing of menstrual cycle and chronic anovulation while, in older women obesity, IR, and metabolic disturbances are predominant.[24] An association between younger age at menarche and development of PCOS was observed in adolescents (odds ratio: 0.63 [95% CI: 0.47; 0.85] p = 0.003).[25] Thus, careful consideration must be given to the age of puberty and presentation of PCOS; deviations in terms of early or late puberty may be a risk factor for development of PCOS. Cutaneous manifestations like early acne or hirsutism, persistent acne and hirsutism for > two years, persistent severe acne; frequent relapse in acne; acne in facial V area are also known to be associated with PCOS [Figure 1].[26,27]
Compared to women with PCOS of Caucasian ethnicity, Indian women with PCOS have a higher degree of hirsutism, infertility, and acne; and experience lower live birth rates following in vitro fertilization.[28] Similarly, South Asians with PCOS have a higher prevalence of IR and MS compared to BMI matched PCOS patients from other ethnic groups (Definition: Appendix I).[28]
A rapid increase in the prevalence of PCOS associated morbid conditions such as IR, excess body fat, adverse body fat patterning, hypertriglyceridemia, and obesity-related disease (diabetes and CVD) in Asian Indians has been noted in a recent review of literature on PCOS.[7] Thus, in patients of South Asian and specifically Indian ethnicity, regular PCOS surveillance is warranted.
Existing Guidelines:
Currently, no strategy for stratifying the risk of PCOS in the general population has been suggested by any major guidelines. The clinical practice guidelines from Endocrine society, USA,[29] PCOS Australian alliance, Australia,[30] The Royal College of Obstetricians and Gynecologists (RCOG), UK,[31] and Society of Obstetricians and Gynecologists of Canada (SOGC), Canada[32] have not proposed a system of risk classification in general population. However, in Indian clinical practice a preliminary assessment of risk in general population is likely to help in further referrals to higher medical centers for appropriate diagnosis and management. Such a risk classification is only to help in a preliminary assessment and not to posit an alternative scheme of diagnosis; in primary care settings such a risk classification is likely to help in proper channeling of patients to specialized centers for systematic diagnosis.
Recommendations on risk factors for assessment of PCOS
-
It is recommended that Indian women showing at least one biochemical characteristic in conjunction with one clinical symptom should be considered for further evaluation for the likelihood of PCOS (Grade A, EL 3).
Biochemical characteristics: high BMI for overweight/obesity >23 kg/m2 for adults and > 97.5 th percentile for age in adolescents), insulin resistance (aconthosis nigricans as clinical marker of insulin resistance), family history of diabetes or PCOS, obesity and inadequate lifestyle, any marker of lipid metabolic dysregulation (elevated serum total cholesterol, triglyceride and LDL-C levels),
Clinical symptoms: pubertal deviations (early or late), disturbances in periodicity/ timing of menstrual cycle, presence of PCO and clinical signs of hyperandrogenism such as early acne or hirsutism, persistent severe acne, frequent relapse in acne, acne in facial 'V' area, persistent acne and hirsutism for more than two years
In women suspected to have PCOS, it is recommended to screen and appropriately document all clinical and biochemical risk factors in the case history (Grade A, EL 4).
It is recommended that patients who currently show either a clinical symptom or fit into a biochemical characteristic may be referred for further diagnosis when feasible or should be regularly monitored for appearance of other presentations of PCOS (Grade A, EL 4)
It is recommended that individual patients with two or more clinical risk factors be subjectively assessed by the gynecologist and referred to an appropriate healthcare provider for further diagnosis of PCOS (Grade B, EL 4).
DIAGNOSTIC CRITERIA FOR ADULTS AND ADOLESCENTS WITH PCOS
The three main criteria for diagnosis of PCOS are androgen excess (AE), chronic anovulation, and presence of (PCO)
[Table 1].[2,29,33,34] Initially, hyperandrogenism (clinical or biochemical) and anovulation along with recommendations for exclusion of other mimicking etiologies were common diagnostic criteria.[2,33,34] The European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) sponsored Rotterdam group on PCOS introduced an extension to incorporate ovarian morphology (based on an ulatrasonogram).[2] The Rotterdam criteria proposed that a positive observation of two of the three criteria (AE, ovulatory dysfunction, or PCO) constituted a diagnosis for PCOS. A recent National Institute of Health (NIH)-sponsored workshop on PCOS endorsed the Rotterdam criteria for diagnosis of PCOS.[35]

The diagnostic criteria proposed for diagnosis of PCOS are established in adults, and requires objective evaluation for diagnosis in adolescents.
Current evidence
Androgen excess
It is established using either clinical or biochemical determination of hyperandrogenism.[2]
Biochemical hyperandrogenism
Biochemical hyperandrogenism is a measure of androgen levels, determined by total, bioavailable, or free serum testosterone (T) levels.[36] In addition, the free androgen index (FAI=100 x [total testosterone/ Sex hormone-binding globulin]) is also widely used.[30] However, total serum T levels are considered more reliable and suitable for diagnosing androgens status.[3,37] Given the methodological challenges, variability in T levels during pubertal development,[38] and uncertainty in clinical practice[39] defining absolute values using local assays that are diagnostic of PCOS and/or to exclude other causes of hyperandrogenism is preferable. Further, since the physiological T levels alter with pharmacological agents used for induction of periods, diagnosis of AE should not be carried in these subjects.
Clinical hyperandrogenism
Clinical hyperandrogenism includes hirsutism, acne, and androgenic/ central alopecia.[30]
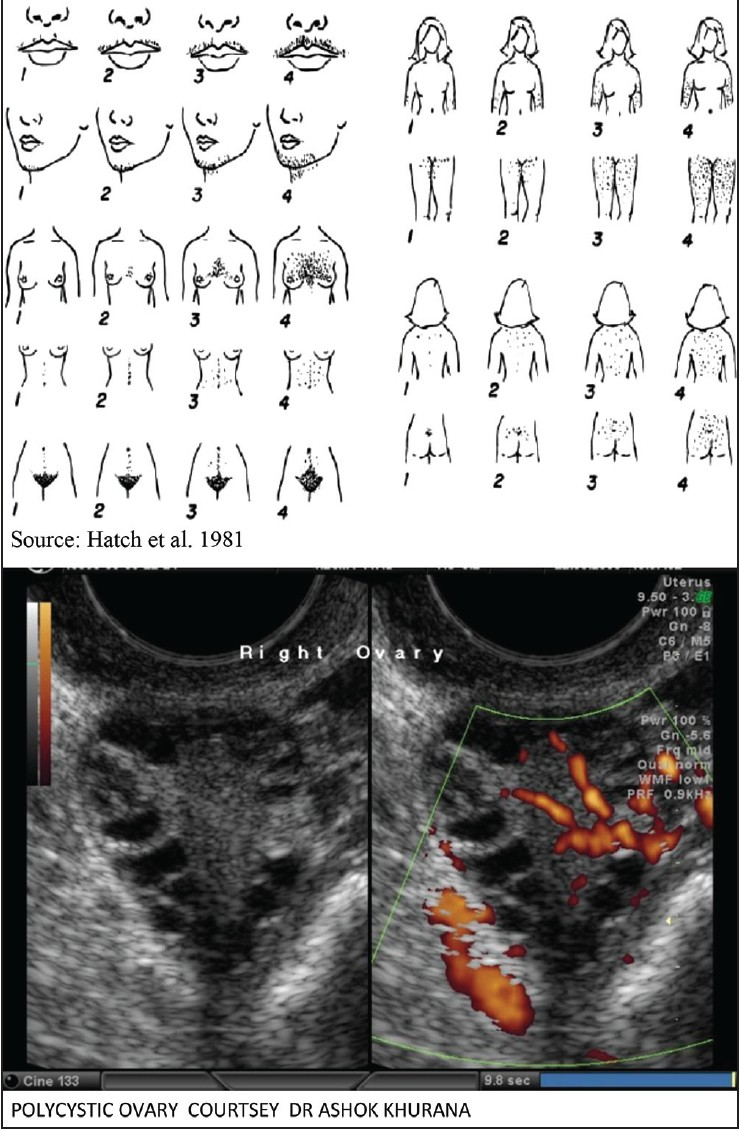
A. Hirsutism: Hirsutism is the excessive growth of thick, dark terminal hair in women where hair growth is normally absent.[40] The modified Ferriman-Gallwey (mFG) score is used to grade hirsutism,[41] which is also used in India.[37,42,43,44] A score of 0 (none) to 4 (severe) in nine areas of the body is assigned with a maximum possible score of 36. Scores < 4 indicate mild hirsutism, 4-7 indicate moderate hirsutism, ≥ 8 indicate severe hirsutism.[40,41] In addition, presentation of AN with or without obesity is suggested an additional diagnostic criteria in adults and adolescents [Figure 2].

- Treatment algorithm for acne, based on endocrine evaluation-Recommendation of Indian Acne Association
B. Acne: Acne can be graded as mild, moderate, and severe forms of acne, based on the number and types of inflammatory lesions [Appendix Table 1]. The prevalence of acne varies in relation to age and ethnicity. In girls, acne starts between 12-14 years of age, and in boys between 14-16 years of age.[45] A description of the location and type of acne lesions according to the age group, as described by the Indian Acne Association (IAA) is presented in Appendix Table 2.
Among the different variants of acne, the SAHA syndrome (seborrhea, acne, hirsutism, alopecia) denotes acne specific to endocrine abnormality of a subject. When diagnosing PCOS in adolescents, acne, a common and transient feature[46] should not be interpreted in isolation, as is the case with androgenic alopecia.[47]
C. Alopecia: Androgenic/central alopecia may also be presented as female pattern hair loss in some patients with PCOS. Ludwig score is used to grade androgenic alopecia.[48]
Ovulatory dysfunction
Ovulatory dysfunction is assessed by menstrual history of oligo/anovulation with bleeding intervals outside the normal interval (25-35 days), happening frequently at ≤ 21 days and/or infrequently at ≥ 35 days. In adults with regular cycles and anovulation, the Rotterdam criteria suggest determining anovulation with mid-luteal progesterone test to help diagnose PCOS.[1] The presence of oligomenorrhea during normal reproductive maturation in adolescents must not be confused with PCOS.
In adolescents, anovulatory cycles comprise 85%, 59% and 25% during the first, third and sixth years, respectively of normal puberty after menarche. Therefore, determining anovulation with mid-luteal progesterone test might help diagnose PCOS in adolescents, and is a matter of clinical debate. The high serum androgen and leutinizing hormone (LH) levels, occurring naturally during anovulatory cycles of adolescence, might not be sufficient to diagnose PCOS in them.[49] A persistent observation of oligo-/amenorrhea beyond two years of menarche in children/adolescents can be evaluated as an early clinical sign of PCOS.[50,51]
Polycystic ovary
Polycystic ovary morphology as defined by ESHRE/ASRM consensus criteria is as at least one ovary with ≥ 12 follicles of 2-9mm (between day 2-5 of cycle) or ovarian volume > 10mL in the absence of a cyst or dominant follicle > 10 mm,[52] established with ultrasound examination of ovaries. It is also endorsed by the Rotterdam criteria[2] and NIH.[35] It is important to distinguish PCO from multi-follicular ovaries to make an appropriate diagnosis of PCO morphology.[2,29] Multi-follicular ovaries contain larger (up to 10 mm diameter) and fewer (up to 6 each ovary) cysts, without hypertrophic echogenic stroma.[53] A study comparing sonographic ovarian morphology in Indian women with and without PCOS found that a combination of two-three sonographic criteria is required to improve the sensitivity of PCO diagnosis.[54]
In normal adolescent physiology, presence of multi-follicular/ PCO is a common feature that decreases with cycle regularity[55] and requires strict interpretation of ultrasonography findings of PCO morphology.[56] Since no well-defined cut-off values for determining the levels of anti-mullerian hormone (non-invasive test) exist, ultrasound examination of ovaries is recommended for the diagnosis of PCO morphology in adolescents also.[51] In adolescent girls with PCOS, obesity is common; magnetic resonance imaging (MRI) scanning is a more accurate modality[57] to evaluate PCO in these adolescents.[58]
On the other hand, ovarian volume and follicle number decrease from peak reproductive years to age > 40 years in normal as well as PCOS women.[59] Therefore, diagnostic criteria with a combination of age, log ovarian volume, follicle number, and testosterone can be used to distinguish PCO morphology in women at menopausal stage.[59]
Exclusion criteria for diagnosis of PCOS
PCOS is considered a diagnosis of exclusion. During the diagnosis of PCOS, it is important to screen all women to exclude other disorders like thyroid disease, prolactin excess and non-classical congenital adrenal hyperplasia, which mimic the symptoms of PCOS [Table 2].[60,61,62] Mild prolactinemia and subclinical hyperthyroidism are common features in patients with PCOS. Therefore a referral to specialist is required if excess values are reported for prolactin and thyroid stimulating hormone (TSH) T4 levels.

Minimal diagnosis of PCOS in adolescents
Since the three diagnostic criteria for PCOS defined by the guidelines were derived for adults, to establish the diagnosis of PCOS in adolescents, other biochemical and clinical estimations can be ordered by the consulting physician or gynecologist. However, it is essential to order use minimal tests possible to diagnose PCOS in adolescent subjects to avoid burden of tests [Figure 2].
Existing guidelines
The clinical practice guidelines from Endocrine society, USA,[29] PCOS Australian alliance, Australia,[30] The RCOG, UK,[31] and SOGC, Canada[32] endorse the Rotterdam criteria for the diagnosis of PCOS. However, all guidelines advocate that specific phenotypes leading to diagnosis of PCOS be documented in all research studies and clinical care.
Recommendations for the diagnosis of PCOS in adults and adolescents
In women with PCOS, for the objective assessment of cutaneous manifestations such as hirsutism, acne and androgenic alopecia, Indian specific grading should be performed with appropriate scales and possibility of other etiologies should be excluded (Grade B, EL 3)
Adults
-
In adult women, it is recommended that diagnosis of PCOS be made using the Rotterdam criteria, meeting two of the following three conditions: (Grade A, EL 4)
-
Androgen excess
biochemical: serum total testosterone
clinical: persistent acne, hirsutism, female pattern hair loss
Ovulatory dysfunction
Polycystic ovary
-
Presentation of acanthosis nigricans with or without obesity is an additional diagnostic criterion for PCOS in adults and adolescents* (Grade B, EL 4).
Mild prolactinemia and subclinical hypothyroidism are common in PCOS; referral to specialist should be made when indicated by prolactin or TSH, T4 levels (Grade B, EL 4)
Determination of anti-mullerian hormone levels for diagnosis of PCO is not recommended in adult and adolescent women (Grade A, EL 4).
In peri-menopausal and menopausal women with a clinical history of prolonged periods of androgen excess and oligomenorrhea during the reproductive years, additional evidence of PCO morphology, log ovarian volume, follicle number, and testosterone should be considered as a diagnosis of PCOS (Grade B, EL 3).
Adolescents
-
In adolescents, presence of oligomenorrhea or amenorrhea beyond two years of menarche should be considered an early clinical sign of PCOS, followed by Rotterdam criteria (of adults) for diagnosis of PCOS (Grade B, EL 4).
-
Androgen excess
biochemical: serum total testosterone
clinical: acne, hirsutism, female pattern hair loss
Ovulatory dysfunction
PCO with strict interpretation of ultrasonography findings
-
-
Minimal diagnosis of PCOS in adolescents should include 5 tests (Grade A, EL 4):
Serum total testosterone (cut off 60 ng/dL)
OGTT (at zero and two hours after 75 g glucose load)
Serum 17- hydroxy progesterone (assessed at 8 am)
erum TSH
Serum prolactin levels
For the diagnosis of PCOS in adolescents, serum LH, follicle stimulating hormone(FSH) and cortisol should be assessed as indicated (Grade B, EL 4).
*Healthcare provider should assess other signs of IR and MS
MANAGEMENT OF PATIENTS WITH PCOS
Management of PCOS stretches beyond the realm of symptomatic treatment and encompasses management of long-term consequences that have clinical and psychological effects on women with PCOS. Both non-pharmacological and pharmacological management strategies are crucial in overall management of PCOS. Because the three main characteristics of PCOS (hyperandrogenism, oligoovulation and IR) drive most of its long-term consequences, management approaches targeted at them may potentially provide improvement in all aspects of the syndrome.
Non-pharmacological interventions for management of obesity and body weight in patients with PCOS
Management of IR and obesity should be considered the first-line of treatment of PCOS. A meta-analysis reported improved levels of FSH, sex-hormone binding globulin, total T, androstenedione, FAI, and mFG score in women with PCOS as a result of lifestyle interventions (diet and physical activity); similar improvements in metabolic indicators were also reported.[63,64,65]
Exercise
Current Evidence
Studies on PCOS from India reported a prevalence rate of 37.5%[66] to 62.5%[37] for obesity in patients with PCOS. A family history of obesity is also associated with PCOS phenotype.[67] Obese women with PCOS have a higher incidence of characteristics of MS (hypertension, impaired glucose tolerance (IGT) and type 2 diabetes Mellitus (T2DM) as well as higher odds of irregular menstrual cycles and clinical hyperandrogenism than lean women with PCOS.[66] Despite lack of large RCTs, the benefits of physical activity (at least 150 minutes of per week) in improving metabolic status and reducing the incidence of diabetes in high risk groups of general population have been demonstrated in small controlled studies.[68,69]
In Indian adolescents with PCOS, compared to controlled (C) treatment with physical exercise, holistic yoga (Y) was found to significantly reduce the T levels (Y=-6.01, C=+2.61, p = 0.014), mFG score for hirsutism (Y=-1.14, C=+0.06, p = 0.002), and improved menstrual frequency (Y=0.89, C=0.49, p = 0.049).[70]
Another RCT on adolescents with PCOS from India found significantly improved fasting insulin and glucose levels, HOMA-IR, and lipid values, independent of their anthropometric changes, with yoga practice compared to conventional physical exercise.[71]
Existing guidelines
Clinical practice guidelines from Endocrine society[29] and RCOG[31] suggest exercise therapy in the management of weight and obesity in PCOS.
Recommendations on non-pharmacological management of PCOS- physical activity
In adults and adolescents with PCOS, daily strict physical activity sessions for at least 30min/day or 150min/ week are recommended (Grade A, EL 4).
Diet
Current evidence
While the benefits of diet control on obesity and IR in PCOS have been widely reported, data from RCTs, especially in Indian women is limited. Women with PCOS have been reported to have higher prevalence of central obesity.[72] In women with PCOS and obesity, weight loss through diet control has been shown to improve pregnancy rates, normalize hyperandrogenemia,[64,73,74] improve insulin sensitivity, menstrual functions, and hirsutism.[73,75]
However, no PCOS- specific diet has been reported. Therefore, it is essential to consult dietician for optimal weight management in women with PCOS. In patients with weight loss response after lifestyle modification + calorie-restricted diet as first-line therapy, weight neutral, insulin sensitizer drugs such as metformin can be used as second-line therapy.
Existing guidelines
The clinical practice guidelines from Endocrine society,[29] RCOG[31] and PCOS Australia alliance,[30] suggest using low calorie diet as first-line therapy for the management of obesity in PCOS.
Recommendations on non-pharmacological management of PCOS- dietary
For the management of obesity in adults (BMI > 23 kg/m2) and adolescents (BMI > 97.5 th percentile for age) with PCOS, it is recommended to follow lifestyle modifications in combination with healthy, balanced diet consisting of regular, calorie-restricted meals (Grade B, EL 4).
In adult and adolescent women with PCOS, it is recommended to routinely screen for BMI and waist circumference as an index for increasing adiposity and development of hyperandrogenism (Grade A, EL 3).
It is recommended to follow calorie restricted diet (low carbohydrate and fat, high protein) in consultation with dietician and lifestyle modification as first-line therapy for at least 6 months, then add metformin as second-line therapy (Grade B, EL 4).
PHARMACOLOGICAL INTERVENTIONS FOR MANAGEMENT OF PATIENTS WITH PCOS
As discussed above, the choice of treatment in women with PCOS can be broadly categorized to treat the symptoms of menstrual irregularities (MI) and hyperandrogenism.
Menstrual irregularity
Current evidence
In women with (MI), proliferation of endometrium can be inhibited using either cyclic progestin or combined oral contraceptives (COCs: estrogen + progestin). Low-dose COCs (< 50 mcg of estrogen in combination with a progestin) have been the mainstay of treatment for MI in patients with PCOS not willing to conceive. In order to reduce the risk of endometrial proliferative disorders, progesterone withdrawal bleeds are generally accepted as first-line therapy to ameliorate cycle regularity in women with PCOS. Three issues have to be considered while choosing a COC: type of progestin compound used, type of estrogen compound used (usually 30 mcg ethinyl estradiol [EE]), and dosage of progestin and estrogen compounds in combination.[76]
Current evidences from India in the management of MI in women with PCOS are available on COCs with two progestin components- drospirenone and desogestrel. The effects of two COCs 3 mg drospirenone vs. 0.15 mcg desogestrel (in combination with 30 mcg EE) on MI in women with PCOS were compared for 6 month treatment period and 6 months post-treatment.[42] Although patients from both groups achieved menstrual regularity during the treatment, higher proportion of patients from drospirenone group continued to have regular cycles (44.8%) than desogestrel group (17.2%) at 6 months post-treatment (p < 0.01). In another study, drospirenone group was effective in reducing the hirsutism score in patients with both MI and.[42] Overall, drospirenone containing COCs are more efficient compared to desogestrel containing COCs because of its anti-androgenic effects on menstrual cycle regularity, lipid profile, Blood Pressure(BP), and hormonal profile.[42] Besides drospirenone and desogestrel, other progestins commonly used in India for clinical practice, either as cyclic progestin or COCs, for the management of MI in women with PCOS include natural micronized progesterone, dienogest, nor-ethisterone and the levonorgestrel- intrauterine system (LNG-IUS).
Since there is limited evidence of use of COCs in adolescents with MI, physician discretion is needed to judge the long-term effects of estrogen component during normal pubertal development. With clinical experience on patients with PCOS pan-India, the expert panel has suggested use of only low-dose COCs for short-periods (up to7 days) to attain MI in 12-16 year old patients. In this age group, MI was defined as achievement of at least 4 cycles/ year. Similarly, in adolescents above 16 years of age, use of low-dose COCs is permissible for the management of MI.
The AE in women with PCOS is also linked to IR and consequent hyperinsulinemia,[77] driving the use of insulin sensitizers such as metformin[78] and thiazolidinediones[79] in the management of PCOS. The effects of metformin on glucose homeostasis and improved cycle pattern are mainly attributed to increased insulin sensitivity.[80] Metformin in combination with a low-dose anti-androgen (spironolactone) was more beneficial than either drug alone in improving MI in adult women with PCOS, presenting with oligo-/amenorrhea, hyperandrogenism and PCO morphology.[81,82]
However, metformin monotherapy for six months resulted in regular menses within 4 months of treatment, but a consistent reversal towards pre-treatment conditions was observed within 3 months of metformin withdrawal.[83] Adverse events such as vomiting, nausea, diarrhea, and hyperadrenergic symptoms were reported in patients taking metformin including drug withdrawal in subjects.[81] Therefore the expert panel recommends against the use of metformin as first-line therapy for MI, but as second-line therapy with or without low-dose COCs, if COCs are not successful or tolerated.
The duration of metformin treatment for treatment of ovulatory dysfunctions in adolescents is not established due to very limited evidence from long-term studies and conflicting evidences from short-term studies. Therefore an extrapolation of evidence from studies conducted in adults may be required to recommend the use of metformin in adolescents. Use of spironolactone alone for the management of MI seems clinically inappropriate, since MI have been reported in 18% patients with low-dose spironolactone (50-100 mg daily) and in 70% patients with high-dose (200 mg daily).[84,85] In an Indian study, polyuria (> 5%), abdominal pain, MI (> 10%), and dryness of mouth were reported in patients with PCOS taking spironolactone, and caused drug withdrawal in four subjects.[81]
Existing guidelines
The ACOG recommends low-dose COCs as primary treatment option for improved MI and other menstrual disorders in women with PCOS.[86] The clinical practice guidelines from Endocrine society,[29] RCOG[31] and PCOS Australia alliance,[30] also recommend use of hormonal contraceptives as first-line therapy for menstrual abnormalities of PCOS. The guidelines from Endocrine society[29] further recommend screening contraindications to COC use via established criteria set by 'Centers for disease control and prevention (CDC)- US medical eligibility criteria for contraceptive use'.
Recommendations on management of menstrual irregularity in PCOS [Figure 2]
Adults
In adults with PCOS showing menstrual irregularity, it is recommended to include progesterone withdrawal bleeds as first-line therapy till menopause to avoid the risk of endometrial proliferative disorders (Grade A, EL 4)
In adults with PCOS who do not intend to conceive, it is recommended to use COCs (drospirenone and desogestrel as progestin component) for the management of menstrual irregularity (Grade A, EL 1). Drospirenone has been shown to be more beneficial than desogestrel in Indian conditions.
In women with PCOS, metformin is not recommended as first-line therapy for the management of menstrual irregularity (Grade A, EL 4).
In women with PCOS, spironolactone is not recommended for menstrual irregularity (Grade B, EL 4)
In adults and adolescents with PCOS, if there is no improvement of menstrual irregularity with COCs or COCs are not tolerated, it is recommended to use insulin sensitizers such as metformin (with or without progestins), but not thiazolidinediones for the management of menstrual irregularity (Grade A, EL 2).
Adolescents
In adolescents with PCOS, it is suggested to use low-dose COCs (with or without anti-androgenic progestins- drospirenone and desogestrel) for the management of MI (Grade A, EL 4).
Between 12-16 years of age, low-dose COCs only to be used, for short period (up to 7 days)
After 16 years, low-dose COCs to be used
enstrual regularity: 4 cycles/year in adolescents of 12-16 years
In adults and adolescents with PCOS with menstrual irregularity and hirsutism, low-dose COCs are suggested (Grade A, EL 2).
Hyperandrogenism
Current evidence
Hirsutism, acne and androgenic alopecia are the clinical symptoms of hyperandrogenism observed in women with PCOS. The AE in women with PCOS is manifested as excessive terminal hair growth and acne.[87] A prevalence of 44.16% for hirsutism and AN and 20% for acne were observed in PCOS women from India.[37] Features of clinical hyperandrogenism- hirsutism (33.6% vs. 28%) as well as acne and oily skin (40.6% vs. 22.6%) were found to be significantly higher in obese women with PCOS than lean PCOS women.[66]
Management of hirsutism
Management of hyperandrogenism requires long-term and multi-dimensional treatment. This involves a combination of lifestyle modification, mechanical hair removal methods and pharmacological therapy for androgenic suppression.
Lifestyle modifications
A recent review comparing minimal or no treatment with lifestyle modifications (diet, exercise, behavioral or combined treatments) in patients with PCOS, reported improved body composition, hyperandrogenism and IR in women with PCOS.[18] Further, metformin + weight reduction therapy are reported to reduce IR and T levels in women with PCOS.[88]
Pharmacological therapy
As with menstrual cycles, COCs are first-line agents for pharmacologic treatment of hirsutism in women not willing to conceive.[76] COCs with anti-androgenic progestins such as cyproterone acetate (CPA), drospirenone, desogestrel are generally used for the management of hirsutism in women with PCOS. Parallel administration of direct (mechanical) hair removal methods ameliorates the condition and reduces the time required.
Treatment with two mg CPA was shown to significantly reduce mean FG scores from (14.3 to 5.7) after 12 weeks of therapy in women with PCOS.[89] In another study, two mg CPA + 35 mcg EE (for 48 consecutive cycles) demonstrated significant reduction of mFG score (mean FG score 10.4) in 73% subjects.[90] No significant side effects or patient withdrawal were reported during 48 cycles of therapy, probably due to considerable effects on hirsutism, complete remission of acne, excellent cycle regularity and endometrial control observed with EE/CPA.[90]
Evidence from India comparing COCs: CPA, desogestrel (deso), and drospirenone (dros) in women with PCOS reporting MI+hirsutism, found that CPA showed the strongest anti-androgen activities with significant decrease in mFG score (treatment difference: CPA -5.29, dros -2.12, deso -1.69) after 12 months of treatment.[91] In this study, very few patients reported adverse events: desogestrel group- bloatedness and sensation of weight gain, nausea and headache, rise of BP; CPA group- breast tenderness and absence of withdrawal bleeding in the pill-free week; drospirenone group- one patient with nausea, vomiting, and vertigo, another with altered liver function test. Studies on drospirenone (3 mg + 30 mcg EE)[92] and desogestrel (150 mcg +30 mcg EE)[91] also demonstrated significant improvement in mFG score (4.6 vs. 6.4) compared to baseline.
In another evidence from India, compared to metformin, spironolactone demonstrated better improvement in hirsutism score (12.5 ± 4.9 and 12.9 ± 3.2 at baseline to 10.0 ± 3.3 and 8.7±1.9, respectively) after 6 months therapy in women with PCOS reporting MI, hirsutism.[93] Whereas finasteride (5 mg/day), a 5α-reductase inhibitor, in comparison with CPA (25 mg/day on days 5-14) + EE (20 mcg/day on days 5-25) was equally effective in reducing mFG scores after 9 months of treatment.[94] However, due to the risk of teratogenicity (feminization of male infant) with their use,[95,96] the expert panel has recommended to cease the use at least 6 months before planned pregnancy. Therefore, spironolactone and finasteride can be used as second-line treatment for the management of hirsutism in patients with PCOS.
Insulin sensitizers
In addition to hormonal therapy, administration of insulin sensitizers can improve the hyperinsulinemic as well as hyperandrogenic state in women with PCOS. However, due to limited evidence on use of metformin in adolescents without established glucose intolerance, the expert panel recommends against its use in adolescents with PCOS. Further, lifestyle modification is better than metformin in improving hyperandrogenism, obesity and signs of IR. Therefore the expert panel recommends lifestyle modification as first-line therapy followed by metformin in adolescents and children. Metformin should be initiated in children only after a wait-period of two years post-menarche.
By virtue of the nature of hyperandrogenism, the source of androgen cannot be eliminated permanently, and evidence suggests that hyperandrogenism requires long-term therapy. Therefore, ideal time to stop the hormonal therapy for hyperandrogenism cannot be established.
Risk of venous thromboembolism
Use of different COCs with varying risks of venous thromboembolism (VTE) is reported in general population.[97] However evidence in women with PCOS is inconsistent. A recent meta-analysis of trials using either COCs with anti-androgens, or metformin in women with PCOS found that thromboembolic episodes were not reported in any study.[98] A cross-sectional analysis on a database (2003-2008, US women) observed that PCOS women were more likely to have thromboembolism than those without and reported a protective association (OR 0.8; 95% CI: 0.73-0.98) with use of COCs.[99] Since there is lack of consistent data on diagnosis and management of VTE specific to PCOS, the expert panel has suggested ways to minimize the risks and maximize the benefits of COC use. It is essential to regularly monitor the risk and provide three months of pause after one year of COC regimen. Investigations used to monitor VTE risk in general population (such as using duplex ultrasonography for deep vein thrombosis and chest X-ray/ ventilation-perfusion scan/ CT pulmonary angiography for pulmonary embolism) can also be adopted in women with PCOS.[100]
Mechanical hair removal methods
Apart from the pharmacological approaches to deal with hirsutism, temporary and permanent methods of hair removal/ reduction should also be used as first-line therapy for management of hirsutism in women with PCOS. Permanent methods of hair reduction therapy include electrolysis and photo epilation devices such as laser and intense pulsed light. In patients seeking permanent hair reduction therapy, it is essential to initiate pharmacologic therapy to minimize hair regrowth. Temporary hair removal methods such as depilation, epilation, and bleaching are effective in reducing facial hair growth in women with PCOS. In addition, topical treatment with eflornithine, an ornithine decarboxylase inhibitor approved by United states of food and drug administration (US-FDA), is also effective.[76,86,101]
Management of acne
Management of acne needs careful selection of anti-acne agents according to clinical presentation and individual patient needs. Adjunctive therapies of topical applications along with hormone therapy should be used as first-line therapy for synergistic effects. Physical treatment methods (lesion removal, phototherapy) are also suggested for acne management.[102]
Topical applications
Based on the clinical presentation of acne in individual patient, specific topical medication for mild and moderate acne, and maintenance therapy should be prescribed in consultation with dermatologist. Benzoyl peroxide, topical retinoids, and topical antibiotics are used as first-line treatment for acne management.
Hormone therapy
Hormone therapy is suggested as first-line therapy for androgenic acne in women with PCOS, SAHA syndrome, HAIRAN syndrome (hyperandrogenism, IR, AN), or cutaneous hyperandrogenism. The IAA consensus guideline further justifies hormonal therapy in refractory/difficult acne and in nodulocystic acne where isotretinoin is either contraindicated or inadequate.[45] However, due to the multiple causes of acne vulgaris, evaluation of hormonal status is a prerequisite before initiating hormone therapy.[45]
Treatment with two mg CPA (+35 mcg EE) for 12 cycles significantly reduced acne score in all 41% cases (at baseline), with improved facial acne by the end of third cycle and improved thorax and back acne by end of 6th cycle.[89] Treatment with CPA/EE combination for 48 cycles demonstrated a complete recovery of various types of acne lesions (moderate 67%, severe 33% cases at baseline) in all subjects (100%) within 24 cycles of treatment.[90] In a study conducted in Indian women with PCOS, CPA demonstrated numerically higher reduction in acne score (CPA -1.52, drospirenone -1.42, desogestrel -1.41) compared to other pills.[91] CPA has been well studied as an androgen receptor blocking agent, effective in acne management in females.[103,104] Higher doses of CPA have been reported to be more effective than lower dose to treat acne.[102]
In another study, drospirenone (3 mg + 30 mcg EE) containing COC showed significant improvement in acne at six cycles (54.9% vs. 31.4%, p < 0.05) compared to baseline.[43] Although EE/drospirenone[43] was administered only for 6 months, the beneficial effects in reducing hyperandrogenic features of hirsutism and acne were maintained up to 12 months after treatment. No adverse effects were reported. Similarly, desogestrel (150 mcg)+ EE 30 mcg) also showed significant improvement in the incidence of acne (54.1% vs. 23.4%) in women with PCOS from India after 6 cycles of treatment.[44] No adverse effects were reported up to one year with EE/desogestrel also.[44]
The guidelines issued by IAA recommend hormonal therapy in patients presenting acne with symptoms of SAHA syndrome (with or without irregular cycles) or resistance to conventional therapy/ relapse after isotretinoin therapy along with altered endocrine function. Based on the consensus developed by IAA, hormone therapy with low-dose EE/CPA or high-dose CPA or spironolactone are specifically suggested.[45]
In adolescents with PCOS, improvement in acne was observed with the use of oral contraceptives with anti-androgen activity. In a latest randomized cross-over trial, therapy with medroxyprogesterone acetate and CPA for four months each,[105] CPA significantly improved acne score and LH/FSH ratio.[105] Due to the limited evidence on long-term use of COCs for hyperandrogenic features in adolescents, the expert panel suggested using COCs in adolescents based on the clinical presentation of acne, in consultation with a dermatologist.
A summary of studies conducted in India with COCs containing anti-androgen in progestins (cyproterone acetate, drospirenone, and desogestrel) for the management of hyperandrogenism features is presented in Appendix Table 3.
Management of alopecia
Although there is limited evidence on the management of alopecia, COCs and androgen blockers can be used to reduce alopecia. It is essential to determine and exclude etiologies that mimic alopecia. Ina study on alopecia in general population, CPA+EE demonstrated a marked improvement in alopecia (success rate: 55%).[106] In another study, CPA (for 6-9 months) demonstrated a marked reduction in hair loss, hair thinning, and seborrhea in androgenic feminine alopecia.[107]
Existing guidelines
The clinical practice guidelines from Endocrine society,[29] recommend use of hormonal contraceptives as first-line therapy for management of clinical features of hyperandrogenism such as hirsutism/acne in women with PCOS. The consensus guideline from IAA recommends the use of hormone therapy with low-dose EE/CPA or higher doses of CPA or spironolactone.[45]
Recommendations for management of Hyperandrogenism in PCOS
Hirsutism
-
Following options can be used alone or in combination to suit individual patient needs and clinical requirements for the management of hirsutism:
In adult women with PCOS who do not intend to conceive, it is recommended to use low-does COCs with anti-androgen progestin (cyproterone acetate, drospirenone, or desogestrel) for the management of hirsutism (Grade A, EL 1). Cyproterone acetate has been shown to be more beneficial than other progestins in Indian conditions.
Use of direct hair removal methods are recommended along with COCs as fist-line therapy (Grade A, EL 1). If there is no improvement with COCs or COCs are not tolerated, it is recommended to use spironolactone or finasteride (Grade A, EL 2); spironolactone or finasteride are suggested but recommended to stop 6 months before planned pregnancy.
In women with PCOS, if menstrual irregularity and hirsutism are diagnosed, low-dose COCs with anti-androgenic activity (CPA, drospirenone, desogestrel) are suggested (Grade A, EL 2)The ideal time to stop hormonal therapy for hyperandrogenism cannot be established with existing evidence (Grade A, EL 4).
Risk of thromboembolism with use of COCs can be managed by identifying susceptible patients and/or pausing treatment for 3 months after one year of treatment (Grade A, EL 4).
In adolescents/children with hyperandrogenism, obesity and signs of insulin resistance, lifestyle modification is first-line therapy; metformin is second-line therapy with a wait period of 2 years post-menarche in children (Grade A, EL 4).
In adolescents with hyperandrogenism, if glucose intolerance is not established by OGTT, metformin should not be started (Grade B, EL 4).
Due to insufficient evidence, alternative (acupuncture) and complementary therapeutic options (e.g. myoinositol, omega-3 fatty acids) are not recommended for the management of hyperandrogenism (Grade B, EL 4).
Acne
In adults and adolescents with PCOS and acne, it is suggested to use topical medication along with pharmacological interventions based on the clinical presentation of acne as early as possible, in consultation with dermatologist (Grade A, EL 4).
In adults with PCOS, it is suggested to use oral contraceptives (cyproterone acetate, drospirenone, or desogestrel as progestin component) as first-line therapy for management of all types of acne lesions (Grade A, EL 1). Cyproterone acetate has been shown to be more beneficial than other progestins in Indian conditions.
In adolescents with PCOS and acne, it is suggested to use oral contraceptives (cyproterone acetate, drospirenone, or desogestrel as progestin component) based on the clinical presentation of acne, in consultation with dermatologist (Grade A, EL 2).
Alopecia
In women with PCOS presenting with alopecia, COCs and androgen blockers are recommended as first line therapy (Grade B, EL 3).
EVALUATION AND MANAGEMENT OF ASSOCIATED MORBID CONDITIONS
A number of short- and long-term health problems are associated with PCOS. These include short-term psychosocial problems and long-term problems such as T2DM,[108] obesity, CVD, sleep-disordered breathing[109] and increased risk of endometrial cancer (EC) as well as short-term problems such as impaired fertility, complications during pregnancy.[110]
PSYCHOSOCIAL MANAGEMENT
PCOS is strongly associated with reproductive and metabolic implications affecting patients' psychological functioning and satisfaction with life.[8] Psychological implications entail challenges in depression, physical appearance/ feminine identity, eating habits and psychosexual dysfunction with significant impact on quality of life (QoL).[111]
Depression
Current evidence
Increased rate of depressive symptoms compared to non-BMI matched controls[112,113] with prevalence ranging from 28 to 64% (for depression) and 34 to 57% (for anxiety) have been reported in women with PCOS.[114,115] Also, a lower health related QoL[116] and increased risk of mental depression are reported in women with PCOS.[113] In Indian women a 54% prevalence of depression (GHQ28 score ≥ 8) was reported, of whom 72% were obese, 70% had hirsutism, 61% had acne and 56% were infertile indicating a considerable effect on QoL of these women.[117] The Patient Health Questionnaire 9 (PHQ-9) can be used to measure the severity of depression [Appendix Table 4],[118] whereas the generic Short Form-36 (SF-36)[119] and disease-specific polycystic ovary syndrome questionnaire (PCOSQ)[120] are used to assess the health related QoL in women with PCOS [Appendix Table 5].
Existing guidelines
The clinical practice guidelines from Endocrine society[29] and PCOS Australian alliance, Australia[30] suggest screening all women with PCOS for depression and anxiety by history and, if identified, provide appropriate referral and/or treatment.
Recommendations on management of depression in PCOS
In adults and adolescents with PCOS, it is recommended to routinely screen for depression and anxiety with appropriate psychological instruments (Grade B, EL 3).
In patients with PCOS evaluated with depression and/or anxiety, psychological counseling by an appropriate professional is suggested, based on severity of disease (Grade B, EL 4).
Other psychosocial dysfunctions
Current evidence
In patients with PCOS, negative self-image coupled with lower self-esteem owing to their physical appearance that significantly impacts the mood and QoL[121] are observed. Similarly, significantly more patients with PCOS compared to community controls reported eating disorders (12% vs. 4%) and/or social phobia (27% vs. 2%)[122], which may cause medical, psychological, social and occupational difficulties.[123,124] Physical manifestations of PCOS such as hirsutism, obesity, MI and infertility can have a negative effect on the patients' sexual life.[125]
Existing guidelines
The clinical practice guidelines from PCOS Australian alliance, Australia[30] suggest the screening and assessment of patient's psychosocial dysfunctions and if identified, offer appropriate treatment.
6.1.2 Recommendations on management of other psychosocial dysfunctions in PCOS
If a woman with PCOS is positive on screening for any psychosocial dysfunction, the practitioner should perform a more detailed clinical interview (Grade B EL 4).
In those evaluated with any psychosocial dysfunction, appropriate treatment for improvement of quality of life is suggested (Grade B, EL 4).
Type 2 diabetes mellitus
Current evidence
Diagnosis of PCOS confers a 5- to 10- fold increased risk of developing T2DM.[126,127,128] IR, resulting in hyperinsulinemia also plays a role in the pathogenesis of reproductive disorders in women with PCOS.[126,129] The prevalence of glucose intolerance in Indian women with PCOS[93] was reported as 16.3% (adults 19.1%, adolescents 9.7%).[67]
Due to the risk of IGT, T2DM, and IR in PCOS, evaluation of abnormalities in glucose intolerance at periodic intervals is essential.[9,31,82] ()A 75-gm (OGTT with 75 g- oral glucose load) has been recommended over measuring glycated hemoglobin (HbA1c) for the detection of T2DM[130] and fasting glucose[67] for assessment of IGT.[2] An observational study from India reported '2-hour post-glucose insulin levels' as a better indicator of IR in women with PCOS than other techniques.[131]
Lifestyle modification with exercise and diet are the first-line treatment for weight management and impaired IGT in women with PCOS. Due to the inherent IR in this condition, oral anti diabetics (OADs) particularly insulin sensitizers such as metformin are the most promising pharmacological option,[132]. However, an early referral to specialist diabetological care is recommended for timely management of diabetes and its complications. Metformin alone or in combination with low-dose spironolactone/clomiphene has demonstrated significant alleviation of not only the MI but also glucose tolerance and insulin sensitivity in patients with PCOS.[81,133]
Hyperthyroidism
Evidence from India reported high prevalence of thyroid disorders (higher mean TSH level 4.547 ± 2.66 vs. 2.67 ± 3.11, p < 0.05) in women with PCOS than those without.[134] Metformin can lower TSH levels in women with PCOS and hypothyroidism. Metformin (1500 mg/day) was shown to significantly decrease (mean ± SD) TSH levels (7.78 ± 1.74 at baseline to 6.14 ± 2.47, p < 0.001) over placebo in overweight women with PCOS and hypothyroidism after 6 months of treatment, with no significant change in free T3 and free T4 levels throughout the study.[135]
Existing guidelines
The clinical practice guidelines from Endocrine society, USA recommend screening women with PCOS for IGT test and T2DM using OGTT or HbA1c and suggest rescreening based on development of clinical factors and/or symptoms of diabetes.[29]
The RCOG suggests fasting blood glucose test or an OGTT for screening of diabetes in women with PCOS. The clinical practice guidelines from Endocrine society recommend use of metformin in women with PCOS with T2DM or IGT and fail lifestyle modification and suggest the same in adolescent stage if the goal is to treat IGT/MS.[29]
Recommendations for the management of diabetes in PCOS
In women with PCOS who develop symptoms and/or a risk factor of diabetes, screening at a clinically feasible periodicity is suggested (Grade B, EL 4).
It is recommended to screen adult and adolescent women with PCOS for impaired glucose tolerance and T2DM using a 75 gm oral glucose tolerance test; an HbA1c test should be used only when an OGTT is not feasible (Grade A, EL 2).
In women with PCOS who have impaired glucose tolerance or T2DM, it is recommended to use metformin alone, or in combination with oral contraceptives (Grade A, EL 1).
Early referral to specialist diabetological care is recommended for timely management of diabetes and its complications (Grade A, EL 4)
Cardiovascular risk
Women with PCOS often have cardiovascular disease risk factors. The AE-PCOS society recommends all women with PCOS to be assessed for CVD risk; to test for BMI, waist circumference and blood pressure at each clinical visit.[136,137] In women diagnosed with PCOS, a complete fasting lipid profile as well as OGTT (if BMI > 30 kg/m2, age > 40 years, personal history of GDM, or family history of T2DM) are recommended.[136] A stratification of risk factors for CVD in women with PCOS given by AE-PCOS society is presented in Appendix Table 6.
In India a 37.5% prevalence of obesity (BMI > 27.5 kg/m2),[66] high levels of BP (SBP/DBP > 120/ 80 mm of Hg) inform prospective[138] and cross sectional[139] studies were reported in women with PCOS. Further, increased LDL-C, CVD risk factors, markers of dyslipidemia were noted[131,139,140] markers of and atherosclerosis, flow-mediated dilatation of brachial artery (12.18 ± 2.3% in PCOS vs. 8.3 ± 2.23% in control) and carotid intima media thickness (0.68 ± 0.11 in PCOS vs 0.52 ± 0.02 in control, p = 0.01) were reported in women with PCOS compared to control subjects.[139] Epidemiological data from USA reported a relative risk of 1.53 (95% CI: 1.24; 1.90) for CVD (after adjustment for BMI and potential confounders) in women with very irregular cycles.[141] Therefore, in women with PCOS showing CV risk factors, specialist CV monitoring and care is recommended, irrespective of the severity of their symptoms.
Existing guidelines
The clinical practice guidelines from Endocrine society, USA,[29] PCOS Australia alliance, Australia,[30] RCOG,[31] and AE-PCOS society[136] recommend screening women with PCOS for CV risk factors. The clinical practice guidelines from Endocrine society[29] suggest lifestyle modifications as first-line therapy for CVD and metformin as second line therapy in patients failing to achieve weight reduction with lifestyle modification.[29] The RCOG guidelines[31] suggest hypertension should be treated, but recommended against the use of routine lipid-lowering treatment in women with PCOS and risk of CVD. Consultation with specialists has been recommended by other guidelines for prescribing lipid lowering agents.
Recommendations for the management of cardiovascular risk in PCOS
It is recommended to screen for CV disease in adult women with PCOS by assessing risk factors: obesity (especially abdominal obesity), smoking, hypertension, dyslipidemia (increased LDL-C), vascular disease, IGT, high-sensitivity C-reactive protein, homocysteine, and family history of premature CVD (Grade A, EL 1).
It is recommended to screen for CV disease in adult women with PCOS by assessing high risk factors: metabolic syndrome, T2DM, overt vascular or renal disease (Grade A, EL 1).
It is suggested to assess obesity (by BMI and WC), lipid profile, oral glucose tolerance test, and BP in adult women at baseline, and repeat lipid profile and OGTT at 6 months for borderline risk and one year for normal profiles (Grade B, EL 4).
Specialist CV monitoring and care is recommended in all patients showing CV risk factors, irrespective of the severity of their symptoms (Grade A, EL 4).
Pregnancy complications
Current evidence
A population based cohort study among women with PCOS from Sweden observed that PCOS was strongly associated with pre-eclampsia (adjusted OR 1.45, 95% CI: 1.24-f1.69), preterm birth (2.21, 1.69 to 2.90), more than double risk of GDM (2.32, 1.88 to 2.88), and birth of large for gestational age infants (1.39, 1.19 to 1.62).[142] The results are further confirmed in an independent meta-analysis by Kjerulff.[143] Recurrent pregnancy loss (RPL) in women with PCOS is commonly associated with IR,[144] hyperhomocystinemia (HHcy)[145] and obesity.[146] In a retrospective study on RPL, a significantly higher incidence of HHcy (70.63% vs. 57.26%, p < 0.04) and IR (56.34% vs. 6.83%, p < 0.0001) was observed in women with PCOS compared to controls. Further analysis revealed HHcy as a strong plausible factor for diagnosis of RPL than IR (probability percentage: HHcy=43.32%, IR=37.29%). Findings on association of PCOS and GDM are conflicting with respect to BMI. Recent systematic review and meta-analysis in women with PCOS observed a significantly higher risk for development of GDM in PCOS women than those without (OR: 2.89, 95% CI: 1.68-4.98), albeit with significant statistical heterogeneity due to sensitivity analysis (I (2) = 59.3%).[147] An RCT in women with PCOS treated with metformin found no difference in the prevalence of pre-eclampsia (p = 0.18), preterm delivery (p = 0.12), or prevalence of GDM (p = 0.87) compared to controls during pregnancy.[148]
A latest meta-analysis of RCTs (n=5) comparing the effects of metformin with insulin on glycaemic control, maternal and neonatal outcomes in GDM concluded that metformin is comparable with insulin and might be more suitable for women with mild GDM.[149] An observational study from India reported significantly higher benefits of using metformin over insulin in the management of GDM and T2DM.[150] Although metformin is reported to be comparable with or superior to insulin in terms of glycaemic control and neonatal outcomes, lack of strong evidence on use of metformin in Indian subjects restricts its clinical use.
Existing guidelines
The clinical practice guidelines from Endocrine society, USA[29] recommend pre-conceptual assessment of BMI, BP and OGTT whereas RCOG guidelines recommend screening for GDM before 20 weeks of gestation, in PCOS women requiring ovulation.[31] The clinical practice guidelines from Endocrine society[29] and RCOG guidelines[31] suggest against the use of metformin as first-line treatment for pregnancy complications in women with PCOS.
Recommendations for the management of pregnancy complications in PCOS
In women with PCOS planning to have children, it is recommended to screen for markers of obesity, hypertension and IR to reduce the risk of pregnancy related complications (Grade A, EL 3).
In women with PCOS who have experienced a miscarriage, it is suggested to assess serum homocysteine levels for identification and treatment of hyperhomocystenemia mediated repeated pregnancy losses (Grade B, EL 3).
In women with PCOS, it is recommended not to use metformin therapy only during pregnancy until specific evidence on beneficial effects is demonstrated (Grade B, EL 3).
Endometrial cancer
Current evidence
Long-term studies and meta-analyses have observed an increased risk of development of EC in women with PCOS.[151,152,153] The symptoms, oligo-/amenorrhea, hirsutism, and infertility, as well as risk factors of PCOS, obesity and T2DM, are common to EC.[154,155] Evidence for the applicability of transvaginal sonography (TVS) for predicting endometrial hyperplasia has been inconsistent in literature.[156,157,158]
Indian studies on the measurement of endometrial thickness in women with PCOS have routinely used TVS at a cut-off of 4 mm.[159,160,161] In the context of increased risk of EC in PCOS patients with prolonged amenorrhea, abnormal uterine bleeding, obesity and/or diabetes, it is essential to assess endometrial thickness and raise awareness about EC in these women. It has been established that inducing a withdrawal bleed every 3 to 4 months with progestogens can reduce the risk of EC in women with PCOS.[156] However, since hyperinsulinemia is the primary cause of endometrial hyperplasia, use of insulin sensitizers can reduce the hyperplasia in these women. Overall, for timely detection and management of EC, it is appropriate to screen for development of cancer at regular intervals with reference to oncological specialist.
Existing guidelines
The clinical practice guidelines from Endocrine society[29] suggest against routine screening for endometrial thickness in women with PCOS. The RCOG guidelines suggest investigating oligomenorrheic women and absence of normal withdrawal bleeds using local protocols such as ultrasound scan, endometrial sampling and/or hysteroscopy[31] and recommend treatment with progestogens to induce a withdrawal bleed at least every 3-4 months.
Recommendations for assessment and management of endometrial cancer in PCOS
In women with PCOS without abnormal bleeding, routine screening using TVS is not recommended (Grade B, EL 1)
In women with PCOS with unexpected bleeding and spotting, it is suggested to assess endometrial thickness using TVS and report the same to the physicians (Grade B, EL 4).
In women with PCOS and risk of endometrial carcinoma, it is suggested to use progestogens every 3-4 months (Grade B, EL 3).
Regular oncological referrals, for screening at a clinically feasible periodicity, are recommended for timely detection of endometrial cancer (Grade A, EL 4).
Obstructive sleep apnea
Current evidence
Studies have identified a higher incidence of obstructive sleep apnea (OSA) in women with PCOS compared to controls, even after controlling for BMI.[162,163] Fasting plasma insulin levels and glucose-to-insulin ratios were strongest predictors for OSA. Therefore factors other than obesity may be involved in the high prevalence of OSA in women with PCOS. Interestingly, a decreased likelihood of OSA was reported in adult and post- menopausal women taking hormonal contraceptives.[163,164] There is a lack of quality evidence on OSA in women with PCOS from India.
Existing guidelines
The clinical practice guidelines from Endocrine society[29] suggest screening all overweight/ obese women with PCOS for symptoms suggestive of OSA and, if identified, diagnosed using polysomnography and referred for appropriate therapy. The RCOG guidelines suggest an enquiry of snoring and daytime fatigue /somnolence in all women with PCOS, and educate about the possible risks of OSA, and, if identified, offer diagnosis and appropriate therapy.[31]
6.6 Recommendations for of obstructive sleep apnea in PCOS
In adult and adolescent women with PCOS, it is suggested to routinely screen for OSA and insomnolence, if symptoms are suggestive of OSA, investigate using polysomnography and refer to appropriate institution for further therapy (Grade B, EL 4).
Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis
Current evidence
Ethnicity, increasing age and characteristics of MS (obesity, hypertension, dyslipidemia and diabetes) are risk factors of non-alcoholic fatty liver disease/ non-alcoholic steatohepatitis (NAFLD/NASH).[165] A 15-60% prevalence of NAFLD is reported in women with PCOS.[166,167,168] A cross-sectional, hospital-based study of women with PCOS from India reported a 67%, 31% and 35% prevalence of NASH, NAFLD and MS, respectively.[169]
In the absence of other specific factors, serum markers for liver dysfunction (such as alanine amino transferase) may be used to screen PCOS women with co-existent IR and metabolic risk factors. However, because of low specificity and sensitivity of elevated levels of serum alanine amino transferase, diagnosing NAFLD in these women (PCOS with IR and/or MS) may require non-invasive quantification of fibrosis (ultrasound) and liver biopsy.[170] In view of the potential complications of NAFLD and NASH in women with PCOS having IRand/or MS, early identification and management of NAFLD and MS is essential for overall reduction of effects of MS in PCOS women.
In non-diabetic non-cirrhotic NASH patients[171] and pediatric NASH patients,[172] treatment with vitamin E (800IU/day) for 96 weeks demonstrated significant improvement in serum aminotransferase levels, hepatic steatosis, and lobular inflammation (early stage features) but not portal inflammation and hepatic fibrosis (more advanced histologic features). However, based on a meta-analysis[173] that showed that RCTs with vitamin E and other antioxidants were heterogeneous (with respect to type and dose of drug, treatment duration and follow-up, population [pediatric versus adult], and implementation of lifestyle intervention) a recent review noted a firm conclusion on the effect of vitamin E on NAFLD cannot be made.[174]
Existing guidelines
The clinical practice guidelines from Endocrine society[29] suggest against routine screening of NAFLD and NASH but provide awareness of their possibility in women with PCOS.
Recommendations for NAFLD and NASH in PCOS
In adult and adolescent women with PCOS, it is suggested to provide sufficient awareness on symptoms and complications of NAFLD and NASH and carry out appropriate screening in those diagnosed with insulin resistance and/or metabolic syndrome (Grade B, EL 4).
In patients with PCOS and NASH, treatment with vitamin E is preferred and metformin is not suggested for reduction of metabolic syndrome with specialist inputs from a multidisciplinary team (Grade B, EL 1).
SUMMARY
PCOS is an important emergent public health problem in India that represents a unique trans-generational risk of transmission of a variety of systemic chronic diseases. It has not been possible in contemporary Indian clinical practice to formulate a comprehensive response which is commensurate with the scale of problem that PCOS poses. This has been mainly due to a lack of strong public and academic discourse centered on the proper management of this gargantuan, yet ill-recognized problem. A strong evidentiary foundation is the cornerstone on which the academic discourse on PCOS must be based. In addressing a key driver that feeds the inertia surrounding PCOS, the current GCPR seek to fundamentally redefine the paradigms of PCOS care in India. The approach of current recommendations is to provide a strong rationale for harnessing the mutual synergies in a modern multidisciplinary clinical setting to deliver quality PCOS care while providing an evidence-based structure to standardize the approach to PCOS management across treatment settings. It is hoped that the current GCPR will fulfil a key role in helping current clinical practices to transition to a comprehensive PCOS care paradigm in India.
Source of Support:
Nil
Conflict of Interest:
None declared.
Acknowledgements
The authors thank Jeevan Scientific Technology Limited, Hyderabad, India for providing editorial assistance in the development of this manuscript.
REFERENCES
- The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab.. 2004;89(6):2745-2749.
- [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril.. 2004;81:19-25.
- [Google Scholar]
- Prevalence of polycystic ovary syndrome in young women from North India: A Community-based study. Indian. J. Endocrinol. Metab.. 2012;16(Suppl 2):S389-392.
- [Google Scholar]
- A cross-sectional study of polycystic ovarian syndrome among adolescent and young girls in Mumbai, India. Indian. J. Endocrinol. Metab.. 2014;18(3):317-324.
- [Google Scholar]
- Prevalence of polycystic ovarian syndrome in Indian adolescents. J. Pediatr. Adolesc. Gynecol.. 2011;24:223-227.
- [Google Scholar]
- Menstrual irregularity and poly cystic ovarian syndrome among adolescent girls-a 2 year follow-up study. Indian. J. Pediatr.. 2012;79(Suppl 1):S69-S73.
- [Google Scholar]
- Polycystic ovary syndrome in the Indian subcontinent. Semin. Reprod. Med.. 2008;26(1):22-34.
- [Google Scholar]
- Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC. Med.. 2010;8:41.
- [Google Scholar]
- American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr. Pract.. 2011;17(Suppl 2):1-53.
- [Google Scholar]
- Lifestyle changes in women with polycystic ovary syndrome. Cochrane. Database. Syst. Rev. (7):CD007506.
- [Google Scholar]
- The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes. Rev.. 2013;14(2):95-109.
- [Google Scholar]
- Obesity, free testosterone, and cardiovascular risk factors in adolescents with polycystic ovary syndrome and regularly cycling adolescents. Metabolism. 2006;55(4):508-514.
- [Google Scholar]
- Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes. Care.. 2003;26(5):1380-1384.
- [Google Scholar]
- Physical Status: The use and Interpretation of Anthropometry - Report of a WHO Expert Committee. Geneva: World Health Organization; 1995. p. :263-308.
- Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335(7612):194.
- [Google Scholar]
- Polycystic ovary syndrome: What is the role of obesity? In: Allahbadia GN, Agrawal R, eds. Polycystic Ovary Syndrome. Kent, UK: Anshan, Ltd; 2007. p. :157-163.
- [Google Scholar]
- Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch. Intern. Med.. 2006;166:2081-2086.
- [Google Scholar]
- The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum. Reprod.. 2013;28:2276-2283.
- [Google Scholar]
- Correlation between serum lipid profile and carotid intima-media thickness in Polycystic Ovarian Syndrome. Indian. J. Clin. Biochem.. 2008;23(3):262-266.
- [Google Scholar]
- Insulin resistance and cardiovascular risk factors in women with PCOS who have normal glucose tolerance test. Gynecol. Endocrinol.. 2013;29(2):148-151.
- [Google Scholar]
- Insulin resistance, obesity, hypofibrinolysis, hyperandrogenism, and coronary heart disease risk factors in 25 pre-perimenarchal girls age < or =14 years, 13 with precocious puberty, 23 with a first-degree relative with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab.. 2008;21(10):973-984.
- [Google Scholar]
- Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil. Steril.. 2001;75(1):53-58.
- [Google Scholar]
- The degree of cycle irregularity correlates with the grade of endocrine and metabolic disorders in PCOS patients. Eur. J. Obstet. Gynecol. Reprod. Biol.. 2010;149(2):178-181.
- [Google Scholar]
- Polycystic ovary syndrome and intervening factors in adolescents from 15 to 18 years old. Rev. Assoc. Med. Bras.. 2013;59(4):341-346.
- [Google Scholar]
- Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int. J. Dermatol.. 2013;52(3):371-376.
- [Google Scholar]
- Phenotypic expression of polycystic ovary syndrome in South Asian women. Obstet. Gynecol. Surv.. 2013;68(3):228-234.
- [Google Scholar]
- Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab.. 2013;98(12):4565-4592.
- [Google Scholar]
- Evidence-based guideline for the assessment and management of polycystic ovary syndrome. Melbourne: Jean Hailes Foundation for Women's Health on behalf of the PCOS Australian Alliance; 2011. p. :1-127.
- 2007. Long-term consequences of polycystic ovary syndrome. :1-11. RCOG. Green-top Guideline No. 33 http://www.pcos.gr/gr/files/GT33_LongTermPCOS_rcog.pdf (Last assessed on 25 Nov 14).none
- The diagnosis and management of ovarian hyperstimulation syndrome. J. Obstet. Gynaecol. Can.. 2011;33(11):1156-1162.
- [Google Scholar]
- Diagnostic criteria for polycystic ovarian syndrome: towards a rational approach. In: Dunaif A., Given J.R., Haseltine F., Merriam G.R., eds. Current Issues in Endocrinology & Metabolism: Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. p. :377-384.
- [Google Scholar]
- The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil. Steril.. 2009;91(2):456-488.
- [Google Scholar]
- http://prevention.nih.gov/workshops/2012/pcos/docs/PCOS_Final_Statement.pdfnone (Last assessed on 25 Nov 14).none
- A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab.. 1999;84(10):3666-3672.
- [Google Scholar]
- Clinical characteristics of polycystic ovary syndrome in Indian women. Indian. J. Endocrinol. Metab.. 2013;17(1):138-145.
- [Google Scholar]
- Treatment of PCOS in adolescence. Best. Pract. Res. Clin. Endocrinol. Metab.. 2006;20(2):311-330.
- [Google Scholar]
- Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J. Clin. Endocrinol. Metab.. 2007;92(2):405-413.
- [Google Scholar]
- 2013. Hirsutism: Indian Scenario. API India, medicine update. :299-301. chapter 66 thhttp://www.apiindia.org/medicine_update_2013/chap66.pdf (Last assessed 25 Nov 2014)
- Hirsutism: implications, etiology, and management. Am. J. Obstet. Gynecol.. 1981;140(7):815-830.
- [Google Scholar]
- Effect of oral contraceptive containing ethinyl estradiol combined with drospirenone vs. desogestrel on clinical and biochemical parameters in patients with polycystic ovary syndrome. Contraception. 2010;82(2):139-146.
- [Google Scholar]
- Effects of drospirenone pill in Indian women with polycystic ovary syndrome. J. Turkish-German. Gynecol. Assoc.. 2011;12(3):144-147.
- [Google Scholar]
- Effects of ethinyl estradiol and desogestrel on clinical and metabolic parameters in Indian patients with polycystic ovary syndrome. J. Obstet. Gynaecol. Res.. 2012;38(1):285-290.
- [Google Scholar]
- Indian Acne Alliance (IAA). Acne in India: guidelines for management - IAA consensus document. Indian. J. Dermatol. Venereol. Leprol.. 2009;75(Suppl 1):1-62.
- [Google Scholar]
- Adolescent female acne: etiology and management. J. Pediatr. Adolesc. Gynecol.. 2008;21(4):171-176.
- [Google Scholar]
- The diagnosis of polycystic ovary syndrome in adolescents. Am. J. Obstet. Gynecol.. 2010;203(3):201.e1-e5.
- [Google Scholar]
- Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol.. 1977;97(3):247-254.
- [Google Scholar]
- Diagnosis of the polycystic ovary syndrome in adolescence: comparison of adolescent and adult hyperandrogenism. J. Pediatr. Endocrinol. Metab.. 2000;13(suppl 5):1285-1289.
- [Google Scholar]
- Polycystic ovary syndrome in adolescence-a therapeutic conundrum. Hum. Reprod.. 2004;19(5):1039-1042.
- [Google Scholar]
- Clinical expression of polycystic ovary syndrome in adolescent girls. Fertil. Steril.. 2006;86(Suppl 1):S6.
- [Google Scholar]
- Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum. Reprod. Update.. 2003;9(6):505-514.
- [Google Scholar]
- Multifollicular ovaries: Clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2(8469-70):1375-1379.
- [Google Scholar]
- Comparative evaluation of sonographic ovarian morphology of Indian women with polycystic ovary syndrome versus those of normal women. Indian. J. Endocr. Metab.. 2014;18(2):180-184.
- [Google Scholar]
- Standards for ovarian volume in childhood and puberty. Fertil. Steril.. 1993;60(3):456-460.
- [Google Scholar]
- Guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab.. 2009;94(5):1509-1517.
- [Google Scholar]
- Ovarian imaging by magnetic resonance in obese adolescent girls with polycystic ovary syndrome: a pilot study. Fertil. Steril.. 2005;84(4):985-995.
- [Google Scholar]
- Reproductive endocrinology of adolescent polycystic ovary syndrome. BJOG. 2010;117(2):150-155.
- [Google Scholar]
- Criteria for Polycystic Ovarian Morphology in Polycystic Ovary Syndrome as a Function of Age. J. Clin. Endocrinol. Metab.. 2009;94(12):4961-4970.
- [Google Scholar]
- American Thyroid Association guidelines for detection of thyroid dysfunction. Arch. Intern. Med.. 2000;160(11):1573-1575.
- [Google Scholar]
- Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab.. 2011;96(2):273-288.
- [Google Scholar]
- Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab.. 2010;95(9):4133-4160.
- [Google Scholar]
- Infertility treatment in PCOS: lifestyle Interventions, medications and surgery. In: Macut D, Pfeifer M, Yildiz BO, Diamanti-Kandarakis E, eds. Polycystic ovary syndrome. Basel, Switzerland: Karger; 2013. p. :128-42.
- [Google Scholar]
- Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum. Reprod.. 1998;13(6):1502-1505.
- [Google Scholar]
- Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Endocr. Connect.. 2014;3(1):36-46.
- [Google Scholar]
- Comparison of clinical features and health manifestations in lean vs. obese Indian women with polycystic ovarian syndrome. J. Hum. Reprod. Sci.. 2009;2(1):12-17.
- [Google Scholar]
- Abnormal glucose tolerance in polycystic ovary syndrome. J. Obstet. Gynaecol. Res.. 2008;34(2):228-232.
- [Google Scholar]
- Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med.. 2002;346(6):393-403.
- [Google Scholar]
- Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab.. 2007;32(1):76-88.
- [Google Scholar]
- Effects of a holistic yoga program on endocrine parameters in adolescents with polycystic ovarian syndrome: a randomized controlled trial. J. Altern. Complement. Med.. 2013;19(2):153-160.
- [Google Scholar]
- Effect of a yoga program on glucose metabolism and blood lipid levels in adolescent girls with polycystic ovary syndrome. Int. J. Gynaecol. Obstet.. 2012;118(1):37-41.
- [Google Scholar]
- Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian. J. Endocrinol. Metab.. 2013;17(4):672-676.
- [Google Scholar]
- Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol (Oxf). 1992;36(1):105-111.
- [Google Scholar]
- Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur. J. Endocrinol.. 2011;164(1):53-60.
- [Google Scholar]
- Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab.. 2003;88(2):812-819.
- [Google Scholar]
- Treatment options for polycystic ovary syndrome. Int. J. Womens. Health.. 2011;3:25-35.
- [Google Scholar]
- Sensitization to insulin induces ovulation in nonobese adolescents with anovulatory hyperandrogenism. J. Clin. Endocrinol. Metab.. 2001;86(8):3595-3598.
- [Google Scholar]
- Effects of metformin on body mass index, menstrual cyclicity, and ovulation induction in women with polycystic ovary syndrome. Fertil. Steril.. 2003;79(3):469-481.
- [Google Scholar]
- Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J. Clin. Endocrinol. Metab.. 2001;86(4):1626-1632.
- [Google Scholar]
- Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril.. 2001;76(3):517-524.
- [Google Scholar]
- Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J. Clin. Endocrinol. Metab.. 2013;98(9):3599-3607.
- [Google Scholar]
- Androgen Excess Society. Position statement: criteria for defining Polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J. Clin. Endocrinol. Metab.. 2006;91(11):4237-4245.
- [Google Scholar]
- Sensitization to insulin in adolescent girls to normalize hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism after precocious pubarche. J. Clin. Endocrinol. Metab.. 2000;85(10):3526-3530.
- [Google Scholar]
- Oral Spironolactone in Post-teenage Female Patients with Acne Vulgaris: Practical Considerations for the Clinician Based on Current Data and Clinical Experience. J. Clin. Aesthet. Dermatol.. 2012;5(3):37-50.
- [Google Scholar]
- ACOG Practice Bulletin No 108: Polycystic ovary syndrome. Obstet. Gynecol.. 2009;114(4):936-949.
- [Google Scholar]
- Acne and hirsutism in polycystic ovary syndrome: Clinical, endocrine-metabolic and ultrasonographic differences. Gynecol. Endocrinol.. 2002;16(4):275-284.
- [Google Scholar]
- Body weight reduction and metformin: Roles in polycystic ovary syndrome. Pathophysiology. 2013;20(2):131-137.
- [Google Scholar]
- Results of an open one-year study with Diane-35 in women with polycystic ovarian syndrome. Ann. N. Y. Acad. Sci.. 1993;687:263-271.
- [Google Scholar]
- Efficacy of combined ethinyloestradiol (0.035 mg) and cyproterone acetate (2 mg) in acne and hirsutism in women with polycystic ovary syndrome. J. Obstet. Gynecol.. 1997;17(6):565-568.
- [Google Scholar]
- Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil. Steril.. 2012;98(4):1053-1059.
- [Google Scholar]
- Clinical efficacy and metabolic impact of two different dosages of ethinyl-estradiol in association with drospirenone in normal-weight women with polycystic ovary syndrome: a randomized study. J. Endocrinol. Invest.. 2013;36(8):636-641.
- [Google Scholar]
- Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J. Clin. Endocrinol. Metab.. 2004;89(6):2756-2762.
- [Google Scholar]
- Finasteride versus cyproterone acetate-estrogen regimens in the treatment of hirsutism. Int. J. Gynaecol. Obstet.. 2004;87(1):29-33.
- [Google Scholar]
- A prospective randomized trial comparing finasteride to spironolactone in the treatment of hirsute women. J. Clin. Endocrinol. Metab.. 1995;80(1):233-238.
- [Google Scholar]
- The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009;339:b2921.
- [Google Scholar]
- Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab.. 2013;98(12):4646-4654.
- [Google Scholar]
- Is polycystic ovary syndrome another risk factor for venous thromboembolism? United States, 2003-2008. Am. J. Obstet. Gynecol.. 2012;207(5):377.e1-8.
- [Google Scholar]
- Strategies for diagnosis and prevention of venous thromboembolism during pregnancy. J. Pregnancy.. 2011;2011:206858.
- [Google Scholar]
- Lexi-Comp Online™, Lexi-Drugs Online™. Hudson, Ohio: Lexi-Comp, Inc; 2013.
- Acne vulgaris treatment: the current scenario. Indian. J. Dermatol.. 2011;56(1):7-13.
- [Google Scholar]
- Cypoterone acetate/ ethinyl estradiol in the treatment of acne. A comparative dose-response study of estrogen component. Contraception. 1990;42(2):225-234.
- [Google Scholar]
- A Randomized Crossover Study of Medroxyprogesterone Acetate and Diane-35 in Adolescent Girls with Polycystic Ovarian Syndrome. J. Pediatr. Adolesc. Gynecol.. 2014;27(3):166-171.
- [Google Scholar]
- Treatment of hirsutism, acne and alopecia with cyproterone acetate. Dermatologica. 1980;160(6):398-404.
- [Google Scholar]
- [Assessment of efficacy of Diane-35 in androgenetic feminine alopecia] Wiad. Lek.. 2003;56(3-4):202-205.
- [Google Scholar]
- Polycystic ovarian syndrome (PCOS): a significant contributor to the overall burden of type 2 diabetes in women. J. Womens. Health.. 2007;16(2):191-197.
- [Google Scholar]
- The role of obesity in the increased prevalence of obstructive sleep apnea syndrome in patients with polycystic ovarian syndrome. Sleep. Med.. 2002;3(5):401-404.
- [Google Scholar]
- Pregnancy complications in PCOS. Best. Pract. Res. Clin. Endocrinol. Metab.. 2006;20(2):281-292.
- [Google Scholar]
- Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum. Reprod. Update.. 2008;14(1):15-25.
- [Google Scholar]
- Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom. Med.. 2004;66(3):356-362.
- [Google Scholar]
- Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil. Steril.. 2007;87(6):1369-1376.
- [Google Scholar]
- Anxiety and depression in adolescents with polycystic ovary syndrome and Mayer-Rokitansky-Küster- Hauser syndrome. J. Psychosom. Obstet. Gynaecol.. 2009;30(2):83-88.
- [Google Scholar]
- Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil. Steril.. 2010;93(7):2421-2423.
- [Google Scholar]
- Health-related quality of life issues in women with polycystic ovary syndrome. J. Obstet. Gynecol. Neonatal. Nurs.. 2005;34(1):12-20.
- [Google Scholar]
- Psychosocial aspects of women with polycystic ovary syndrome from south India. J. Assoc. Physicians. India.. 2008;56:945-948.
- [Google Scholar]
- The PHQ- 9: validity of a brief depression severity measure. J. Gen. Intern. Med.. 2001;16(9):606-613.
- [Google Scholar]
- The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care.. 1992;30(6):473-483.
- [Google Scholar]
- Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS) J. Clin. Endocrinol. Metab.. 1998;83(6):1976-1987.
- [Google Scholar]
- Psychological Signs in Patients with Polycystic Ovary Syndrome. J. Family. Reprod. Health.. 2012;6:145-151.
- [Google Scholar]
- Women with polycystic ovary syndrome are often depressed or anxious-a case control study. Psychoneuroendocrinology. 2008;33(8):1132-1138.
- [Google Scholar]
- Eating disorder behaviors are increasing: findings from two sequential community surveys in South Australia. PLoS. One.. 2008;3(2):e1541.
- [Google Scholar]
- Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab.. 2003;88(12):5801-5807.
- [Google Scholar]
- Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab.. 1999;84(1):165-169.
- [Google Scholar]
- Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes. Care.. 1999;22(1):141-146.
- [Google Scholar]
- Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J. Clin. Endocrinol. Metab.. 2002;87(3):1017-1023.
- [Google Scholar]
- Hemoglobin A1c as a tool for the diagnosis of type 2 diabetes in 208 premenopausal women with polycystic ovary syndrome. Fertil. Steril.. 2011;96(5):1275-1280.
- [Google Scholar]
- Efficacy of 2-hour post glucose insulin levels in predicting insulin resistance in polycystic ovarian syndrome with infertility. J. Hum. Reprod. Sci.. 2011;4(1):20-22.
- [Google Scholar]
- The use of oral antidiabetic drugs in the treatment of polycystic ovary syndrome. Zentralbl. Gynakol.. 2003;125(12):507-514.
- [Google Scholar]
- Sequential treatment of metformin and clomiphene citrate in clomiphene-resistant women with polycystic ovary syndrome: a randomized, controlled trial. Hum. Reprod.. 2003;18(2):299-304.
- [Google Scholar]
- Thyroid disorders in polycystic ovarian syndrome subjects: A tertiary hospital based cross-sectional study from Eastern India. Indian. J. Endocrinol. Metab.. 2013;17(2):304-309.
- [Google Scholar]
- Metformin decreases thyrotropin in overweight women with polycystic ovarian syndrome and hypothyroidism. Diab. Vasc. Dis. Res.. 2011;8(1):47-48.
- [Google Scholar]
- Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AEPCOS) Society. J. Clin. Endocrinol. Metab.. 2010;95(5):2038-2049.
- [Google Scholar]
- Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab.. 2008;93(10):3671-3689.
- [Google Scholar]
- Prevalence of ultrasonography proved polycystic ovaries in North Indian women with type 2 diabetes mellitus. Reprod. Biol. Endocrinol.. 2005;3:35.
- [Google Scholar]
- Study of early atherosclerotic markers in women with polycystic ovary syndrome. Indian. J. Endocrinol. Metab.. 2012;16(6):1004-1008.
- [Google Scholar]
- Association of obesity and insulin resistance with dyslipidemia in Indian women with polycystic ovarian syndrome. Indian. J. Med. Sci.. 2006;60(11):447-453.
- [Google Scholar]
- Menstrual cycle irregularity and risk for future cardiovascular disease. J. Clin. Endocrinol. Metab.. 2002;87(5):2013-2017.
- [Google Scholar]
- Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: Population based cohort study. BMJ. 2011;343:d6309.
- [Google Scholar]
- Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am. J. Obstet. Gynecol.. 2011;204(6):558.e1-6.
- [Google Scholar]
- The comparison of insulin resistance frequency in patients with recurrent early pregnancy loss to normal individuals. BMC. Res. Notes.. 2012;5:133.
- [Google Scholar]
- Plasma homocysteine in polycystic ovary syndrome: does it correlate with insulin resistance and ethnicity? Clin. Endocrinol (Oxf).. 2004;60(5):560-567.
- [Google Scholar]
- Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS. One.. 2013;8(5):e64446.
- [Google Scholar]
- Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil. Steril.. 2009;92(2):667-677.
- [Google Scholar]
- Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J. Clin. Endocrinol. Metab.. 2010;95(12):E448-E455.
- [Google Scholar]
- Metformin vs Insulin in the Management of Gestational Diabetes: A meta-Analysis. PLoS. One.. 2013;8(5):e64585.
- [Google Scholar]
- Metformin--a convenient alternative to insulin for Indian women with diabetes in pregnancy. Indian. J. Med. Sci.. 2009;63(11):491-497.
- [Google Scholar]
- Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod. Biomed. Online.. 2009;19(3):398-405.
- [Google Scholar]
- Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum. Reprod.. 2012;27(5):1327-1331.
- [Google Scholar]
- Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum. Fertil (Camb).. 2000;3(2):101-105.
- [Google Scholar]
- Endometrial carcinoma; ovarian dysfunction-a risk factor in young women. Eur. J. Obstet. Gynecol. Reprod. Biol.. 1991;41(2):143-150.
- [Google Scholar]
- Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer. Epidemiol. Biomarkers. Prev.. 2008;17(1):73-79.
- [Google Scholar]
- Ultrasound and menstrual history in predicting endometrial hyperplasia in polycystic ovary syndrome. Obstet. Gynecol.. 2001;98(2):325-331.
- [Google Scholar]
- Value of endometrial thickness measurement for diagnosing focal intrauterine pathology in women without abnormal uterine bleeding. Ultrasound. Obstet. Gynecol.. 2009;33(3):344-348.
- [Google Scholar]
- Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet. Gynecol.. 2010;116(1):160-167.
- [Google Scholar]
- A prospective randomized trial comparing the efficacy of Letrozole and Clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. J. Hum. Reprod. Sci.. 2012;5(1):20-25.
- [Google Scholar]
- Polycystic ovary syndrome: Is obesity a sine qua non? A clinical, hormonal, and metabolic assessment in relation to body mass index. Indian. J. Endocrinol. Metab.. 2012;16(6):996-999.
- [Google Scholar]
- Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J. Hum. Reprod. Sci.. 2012;5(3):262-265.
- [Google Scholar]
- Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab.. 2001;86(3):1175-1180.
- [Google Scholar]
- Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J. Clin. Endocrinol. Metab.. 2001;86(2):517-520.
- [Google Scholar]
- Hormone replacement therapy and sleep-disordered breathing. Am. J. Respir. Crit. Care. Med.. 2003;167(9):1186-1192.
- [Google Scholar]
- A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol.. 2010;53(2):372-384.
- [Google Scholar]
- Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab.. 2006;91(5):1741-1747.
- [Google Scholar]
- Abnormal aminotransferase activity in women with polycystic ovary syndrome. Fertil. Steril.. 2005;83(2):494-497.
- [Google Scholar]
- Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J. Hepatol.. 2007;47(3):412-417.
- [Google Scholar]
- Prevalence of hepatic steatosis in women with polycystic ovary syndrome. J. Hum. Reprod. Sci.. 2013;6(1):9-14.
- [Google Scholar]
- Diagnostic and predictive factors of significant liver fibrosis and minimal lesions in patients with persistent unexplained elevated transaminases. A prospective multicenter study. J. Hepatol.. 2006;45(4):592-599.
- [Google Scholar]
- Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med.. 2010;362(18):1675-1685.
- [Google Scholar]
- Effect of Vitamin e or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents the tonic randomized controlled trial. JAMA. 2011;305(16):1659-1668.
- [Google Scholar]
- A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79-104.
- [Google Scholar]
- Antioxidant therapy in nonalcoholic steatohepatitis. Hepat. Res. Treat.. 2012;2012:947575.
- [Google Scholar]












