Translate this page into:
Spontaneous conception following anti-tubercular treatment for sub-fertile women with multiple imaging markers suggesting genital tuberculosis
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Female genital tuberculosis (FGTB) primarily an asymptomatic disease is one of the most important causes of female infertility in developing countries. Damage to the pelvic organs after genital tuberculosis (GTB) is a well recognized entity. It is thus prudent to diagnose and treat GTB as early as possible to prevent or at least to minimize the damage to the genital organs. Although diagnosis of GTB has been a challenge, its detection and treatment cannot be based on single test and multiple markers must be utilised with the clinical background and early treatment instituted.
Objective:
The present study was aimed to diagnose or predict GTB based primarily on imaging modalities in the form of hysterosalpingography, pelvic ultrasound and supported by the basic laboratory investigations like Mantoux test, Erythrocyte Sedimentation rate (ESR). Once the diagnosis or prediction of GTB was made, an early institution of anti tubercular therapy was done and patients were followed up to observe the spontaneous pregnancy rate.
Material And Methods:
This was a prospective study wherein the patients underwent complete evaluation for infertility including a hysterosalpingograhy and pelvic ultrasonography. The patients were considered to be positive for genital tuberculosis if three or more of the following were found on evaluation: raised ESR (≥ 20mm/first hour), Mantoux test positive (induration ≥ 10 mm), HSG picture or Ultrasonological picture suggestive of GTB.
Observation:
It was seen that out of the 400 women who were included; 265 women (Group A) were adjudged to have genital tuberculosis as per our study protocol and thus were started on ATT whereas 135 (group B) were not put on antitubercular therapy. Within this duration (during or after completion of ATT), 157(59.2%) conceived spontaneously in group A, on the other hand only 27(20%) women conceived spontaneously in group B. This difference was found to be statistically significant (P Value<0.0001).
Conclusion:
Although Genital tuberculosis poses a great diagnostic challenge because of its varied presentations, diverse imaging pictures, and myriads of tests with its own limitations, it is advisable not to resort to all of them. Tests which are simple, feasible, specific and sensitive, and facilitates early diagnosis should be carried out. Institution of anti-tubercular treatment should be done in early disease, thus enhancing the chance of pregnancy and preventing irreversible damage to the genital organs.
Keywords
Antitubercular treatment
genital tuberculosis
imaging markers
INTRODUCTION
Female genital tuberculosis (FGTB) primarily an asymptomatic disease, contributing to a large global burden of extra pulmonary tuberculosis (TB), is one of the most important causes of female infertility in developing countries.[1] While its prevalence is less than 1% in developed nations, it is almost 28% (range 14-41%) in developing countries.[1,2,3] However, it causes a diagnostic dilemma and in most cases, it is inadvertently uncovered during evaluation for infertility. The organ to be most commonly affected is the fallopian tube (92-100%), followed by the endometrium (50%) while the less-frequently affected ones are the: Ovaries (10-30%), cervix (5%), and rarely the vagina or the vulva (<1%).[4,5] If symptomatic, apart from sub-fertility, the disease may present with pelvic pain or menstrual irregularities. As the diagnosis is elusive, even where high index of suspicion exist, an array of tests are resorted to.[6] The diagnosis can thus be established in such cases with the help of various microbiologic, radiologic, and histopathologic tests along with the clinical presentation, but these tests have high specificity but a low sensitivity even in the presence of active TB.[7,8,9]
Damage to the pelvic organs after clinical genital tuberculosis (GTB) is well-recognized both in the presence of active disease as well as during the process of healing and fibrosis. The disease process not only causes tubal obstruction, but also impairs implantation due to endometrial involvement and ovulatory failure from ovarian involvement. Contrary to the fact that tubal involvement is the most predominant, a few reports have found endometrium to be the most commonly involved site.[9] It is thus prudent to diagnose and treat GTB as early as possible during the sub-clinical stage to prevent or at least to minimize the damage to the genital organs. Detection of FGTB by conventional diagnostic methods has been a major challenge. So, the exact diagnosis of FGTB has been found to be contributory by collecting and processing tissue biopsies from endometrium, ovaries, and aspirated fluids for the determination of infection by culture and histopathological examinations. However, these methods have limitations due to their invasive nature, secondary nature of the GTB, low detection rates of infecting organisms, or the lack of the sampled site to be representative of the infected area.[1,10] Similarly, the detection of mycobacterial tuberculosis (MTB) deoxyribonucleic acid (DNA) by TB-Polymerase Chain Reaction (PCR) has shown high sensitivity and specificity for the diagnosis of GTB.[11,12] but it has been found that the number of infertile women in whom the presence of TB was suspected on clinical grounds and also had a positive endometrial TB-PCR test were far less as compared to the number of positives where no clinical ground for suspicion were present.[13] Hence, diagnosis and treatment for GTB cannot be based on single test and multiple markers must be utilised with the clinical background and early treatment instituted. These tests or markers should be simple, easy to perform, inexpensive with minimal reliability on allied facilities. Thus, we aimed to carry out a study to diagnose or predict GTB based primarily on imaging modalities in the form of hysterosalpingography (HSG), pelvic ultrasound, and supported by the basic laboratory investigations like Mantoux test, Erythrocyte Sedimentation rate (ESR). Once the diagnosis or prediction of GTB was made an early institution of anti-tubercular therapy was done and patients were followed up to observe the spontaneous pregnancy rate.
MATERIALS AND METHODS
This was a prospective study conducted at the Assisted Reproductive Technology Center (ART) of Army Hospital, Research and Referral, New Delhi over a period of 1 year: February 2013 to February 2014. One thousand two hundred and sixty-five women were recruited in our study after obtaining a written informed consent.
Inclusion and exclusion criteria
To be a part of the study, the women had to be less than 38 years of age with presence of bilateral patent tubes and a normal semen analysis of the husband. The patients were excluded from the study in the presence of moderate to severe endometriosis, presence of a documented uterine factor in the form of fibroids, polyps, or previous oophorectomy, history of being treated for TB, and those who would not be able to report for follow-up. The primary outcome measure or the objective of the study was to predict genital Kochs on the basis of imaging markers in the form ultrasonography (USG) of the pelvis and a HSG. The secondary outcome measure of the study was to observe the spontaneous pregnancy rate and thus the women with bilateral tubal blockage on HSG or diagnostic laparoscopy if done were excluded from the study protocol; however, the required treatment was instituted. All study group women were subjected to detailed history-taking and clinical examination. Thereafter, hemoglobin estimation, total and differential leucocyte count, Mantoux, Day 2 hormonal profile (Luteinizing hormone, Follicle-stimulating hormone, Prolactin, and Thyroid-stimulating hormone) was carried out. Husbands' semen analysis was also performed to ascertain the male factor.
A HSG was carried out on all study subjects between 8 th -10 th day of the menstrual cycle. A pelvic USG was performed on second day of the periods and repeated again on 14 th and the 21 st day of the cycle. A laparohysteroscopy was not part of the study protocol; however, if the patients had a previous laparohysteroscopy done, the findings were endorsed and taken into consideration for institution of treatment. A premenstrual endometrial biopsy for histopathology, acid-fast bacilli (AFB) culture, and TB-PCR was not performed in any of our subjects as the aim of the study was to diagnose GTB on the basis of clinical suspicion and the imaging modalities in the form of HSG and pelvic USG.
The patients were considered to be positive for GTB if three or more of the following were found on evaluation: Raised ESR (≥20 mm/first hour), Mantoux test positive (induration ≥10 mm), HSG picture, or Ultrasonological picture suggestive of GTB. Differential diagnostic criteria as suggested by Klein et al.[5] for diagnosis of TB on HSG were taken into consideration. They are as follows: Uterine cavity with an irregular contour, bilateral tubal occlusion, visualization of calcified lymph nodes or smaller irregular calcifications in the adnexal area, obstruction of the fallopian tube in the zone of transition between the isthmus and the ampulla, multiple constrictions along the course of the fallopian tube, beading of the tube/tubes, endometrial adhesion, and/or deformity or obliteration of the endometrial cavity in the absence of curettage or of surgical termination of pregnancy [Figures 1-4].
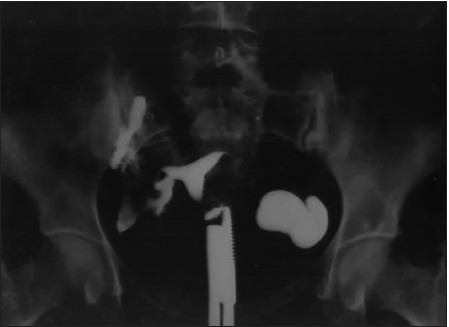
- HSG showing left hydrosalpinx with straightening of tubes on both sides and loculated spill on right side

- HSG showing Irregular filling in uterine cavity suggestive of Asherman's syndrome and bilateral tubal blockage

- HSG showing deformed endometrial cavity

- HSG showing multiple constrictions along the course of the tube
Ultrasonographic findings which were taken as suggestive were: Interruptions in the endometrial extent, endometrial fluid, calcifications in the endometrial cavity, bands or inhomogeneity, a thin endometrium on the 21 st day of the cycle or diffuse variable thickening, sub-endometrial calcification, oligemic myometrial cysts, tubal fluid, free and loculated peritoneal fluid, follicles with echogenic rims, and adnexal fixity [Figures 5-8].

- Irregular endometrium

- USG showing oligaemic myometrial cysts

- USG showing interrupted endometrium with multiple calcifications

- Ultra sound showing obliterated endometrial cavity in the fundal region with thin endometrium on lower part of uterine cavity
Written informed consent was taken from the study group patients and they were counseled for anti-tubercular treatment (ATT) in the event their markers came positive. Of the 1265 women recruited, only 400 were finally included in the study. The reasons for exclusion of the remaining 865 were: Presence of bilateral tubal blockage (215); severe male factor (276); moderate to severe endometriosis (86); previous history of ATT (68); age greater than 38 years (52); previous oophorectomy (62); uterine fibroids (16); history of ectopic pregnancy with unilateral/bilateral salpingectomy (31); and those who did not report for follow-up (52). Of the 400 women assessed for GTB, 265 women were found to have three or more positive finding for GTB as per our protocol and were thus started on the standard four drug ATT. The treatment consisted of the intensive phase of 2 months of four drugs: Isoniazid, (H) 300 mg; Rifampicin, (R) 450-600 mg; Ethambutol, (E) 800-1200 mg, and pyrazinamide, (Z) 1200-1500 mg, followed by the maintenance phase of 4 months comprising the same doses of isoniazid and rifampicin (2HRZE + 4HR). One hundred and thirty-five patients had only two or less features and thus were not started on ATT. Women who had ATT were designated as Group A (n = 265) and women who were not offered ATT were part of group B (n = 135). Patients from both the groups were to follow-up for 1 year. Number of women having a spontaneous pregnancy in each group and time taken to have a spontaneous conception were evaluated and compared statistically.
Statistical analysis
Details of all cases were recorded on a structured format and analyzed with the help of registered version of Statistical Package for the Social Sciences (SPSS) version 13. Group comparisons were made using Chi square (χ2) test (for categorical variables) or Student t-test (for scalar variables). Statistical significance was assessed at P < 0.05
Observation and Results
During the study period, out of the 400 women who were finally included; 265 women were adjudged to have GTB as per our study protocol and thus were started on ATT, whereas 135 were not put on anti-tubercular therapy. The women who formed the part of our prospective study were less than 38 years of age of which, in group A, the mean age of women was 28.8 ± 2.19 years, whereas in Group B, it was 28.4 ± 2.08 years, which was comparable. The other patient characteristics were also comparable in both the groups as depicted in Table 1. Moreover, 82.6% women had primary infertility in the first group and 80% women had never conceived in the second group. The primary complaint in both the study group subjects was sub-fertility with very few having an associated menstrual abnormality. In group A, 21 (7.9%) women had a previous living issue and in group B, 11 (8.1%) had a living issue but there was no statistical difference in the duration of infertility in women from both the study arms.

Of the 265 women who were detected to have GTB as per the study criteria, 184 patients had three features positive for TB and the remaining 81 tested positive for the entire criterion. And 98 of the 184 (54.1%) subjects had features suggestive of GTB in both HSG and USG in comparison to the 86 patients who had either a USG or a HSG feature apart from the hematological parameters [Table 2].

All women irrespective of their treatment status were followed up for 1 year. Within this duration (during or after completion of ATT), 157 (59.2%) conceived spontaneously in group A; on the other hand, only 27 (20%) women conceived spontaneously in group B. This difference was found to be statistically significant (P value <0.0001) [Table 3]. Of the spontaneously conceived women from group A, 11 (4.1%) had an ectopic gestation and all of them were tubal, while 8 (5.9 %) patients from the other group had an extra uterine gestation. Of the eight ectopic gestations which occurred in group B, seven women had a tubal ectopic gestation and one was diagnosed as pregnancy of unknown location. This patient had a falling beta human chorionic gonadotropin (hCG) values over a period of 10 days observation. In group A, the mean duration of time to have spontaneous conception was 6.84 ± 0.81 months; and in group B, it was 7.1 ± 0.92 months.

DISCUSSION
FGTB, an important cause of infertility, primarily affects young women between 18 and 38 years of age. The initial invasion of the genital tract by the mycobacteria occurs with a few bacilli which slowly colonizes and multiplies locally without causing many or overt symptoms. Once an active disease is established, it brings about an irreversible tubal and endometrial damage. However, during the pre-clinical stage, an insidious, low-grade inflammation is likely to occur which alters the function of fallopian tubes, uterus, and of the endometrium and thus leads to subtle damage and partial functional loss of these organs. Molecular mechanisms have also been postulated as an important cause of implantation failure in various gynecologic diseases but not conclusively proven for GTB.[13,14] Nevertheless, mycobacterial infection has been found to alter endometrial milieu and thus the receptivity and cause implantation failure through mechanisms such as disturbed immune-modulation and cytokine over burden, endocrine disruption, activation of anti-phospholipid antibodies and microthrombosis without the presence of overt clinical disease.[14,15,16,17,18] Therefore, any delay in the institution of treatment especially in relation to a vital organ is associated with the risk of permanent loss of function. In pursuance to this, we carried out the present study to diagnose GTB based on various markers and administer anti-tubercular treatment before there would be a permanent loss of function of the reproductive tissues.
A battery of tests: Microbiologic, radiologic, and histopathologic along with the clinical presentation have been utilized to arrive at the diagnosis but each has its own limitations when used in isolation. Thus, multiple markers were taken into account for prediction and administration of drugs. Parikh et al. calculated the prevalence of TB in patients with infertility as 39% based mainly on clinical suspicion and conventional modalities.[19] With routine laboratory tests having little contributory value in the diagnosis and due to the paucibacillary nature of GTB, Thangappah et al. evaluated the efficacy of PCR technique, culture, and histopathological examination in the diagnosis of GTB in female infertility.[13] Their results revealed that conventional methods of diagnosis namely: Histopathological examination, AFB smear and culture have low sensitivity. PCR although useful in diagnosing early disease had a significant false negativity. Therefore, we curtailed the elaborate diagnostic tools for diagnosing GTB and instituted ATT based on simple markers. Of the 400 women who were studied, 265 were considered positive for latent or sub-clinical GTB and started on the standard four drug regimen and 59.2% of them conceived.
Although treatment in the absence of demonstrable tubal damage may be of doubtful benefit to fertility, the presence of mycobacterial DNA demonstrated by a positive PCR indicates infection by tubercle bacilli causing sub-clinical or latent disease potentially responsible for future clinical manifestations. In our study 189 (98 + 81) women had tubal damage which was demonstrated by HSG with varying and specific findings of TB related to tubes such as "beaded tube," "golf club tube," "pipestem tube," "cobble stone tube".[20] Myriads of recent advances in imaging modalities such as computed tomography (CT) scan, magnetic resonance imaging (MRI), and ultrasongraphy have taken place but HSG has been considered as the standard screening test for evaluation of tubal infertility and as a valuable tool for diagnosis of FGTB to assess the outcome of infertility management.[20] In this study too, we had incorporated the HSG as the primary indicator to detect FGTB.
Kulshreshtha et al. in their study compared various modalities for diagnosing GTB and to assess fertility outcome after anti-tubercular therapy (ATT). They had 22.9% spontaneous pregnancies in their study group and concluded that no single test can detect all instances of GTB. A combination of tests is needed to increase the detection rate. It was also observed that treatment given solely on the basis of a positive PCR result can result in conception. However, we did not carry out a PCR but our treatment was based on multiple markers and we had statistically significant spontaneous pregnancy rate in women on ATT.[21] During or after anti-tubercular therapy, 59.2% of our patients became pregnant during a minimum 1-year follow-up period; however, 11 patients had an ectopic pregnancy.[22]
Ultrasound in the diagnosis of female genital TB has not been explored to a great extent but we incorporated the USG findings in our study population. Interruptions in the endometrial extent, endometrial fluid, calcification, bands or inhomogeneity, a thin endometrium or diffuse variable thickening, cornual obliteration, vertical course of the interstitial extent of the tube, impaired endometrial midcycle vascularity in stimulated cycles, sub-endometrial calcification, oligemic myometrial cysts, abnormal midcycle uterine artery flow, tubal fluid, free and loculated peritoneal fluid, inhomogeneous enlarged ovaries, follicles with echogenic rims and adnexal fixity have been postulated to be markers of GTB. FGTB is associated with a wide spectrum of findings on pelvic ultrasound and some of the aforementioned are reasonably specific. This information may be used in determining which patients would benefit from treatment in the absence of microbiological confirmation of disease.[23] Khanna et al. while evaluating markers of GTB in sub-fertile patients observed that pelvic USG revealed irregular endometrial lining in 16 patients, out of which only six (37.5%) were positive for TB-PCR. Eight out of the 21 patients (38.1%) with tubo-ovarian mass were positive for TB-PCR.[22] In the present study, we had taken multiple findings suggestive of GTB apart from irregular endometrial lining and 56% of them had suggestive features.
In our center, we included HSG features, ultrasonographic findings, raised ESR, and positive Mantoux test to diagnose TB. We did not perform DNA PCR of endometrium because of the cost factor which was beyond the affordability of our clients and secondly the test is not performed in our hospital as a routine. High false negativity of DNA PCR is also a limitation of diagnosis, and may thus miss some TB positive cases.[13]
Treatment of asymptomatic or doubtful sub-clinical TB in high-TB prevalence countries such as India can be justified if there is a great risk of progression, disease transmission or loss of function of an organ. Although indications for the treatment of extra-pulmonary TB, mostly paucibacillary, have generally been difficult to conclusively establish, in the absence of definitive criteria for diagnosis, difficulties of obtaining samples and low rates of mycobacterial smear and culture positivity. Nevertheless, most of the studies have shown promising results in the form of spontaneous conception after anti-tubercular therapy. In our study, (59.2%) women conceived with ATT in group A and women who were not put on ATT had conception in only 20% cases. The difference in the spontaneous conception in both group was statistically significant (P value < 0.0001). The mean time for these women to conceive was 5.84 months in contradiction to a study by Suman Puri in which the women with genital TB conceived spontaneously only in 19.2% cases within 2 years of follow-up.[24] In another study conducted by U.N. Jindal, the spontaneous conception rate after ATT was 92.2% within 12 months of ATT.[25] The difference in these studies may be due to the degree and severity of damage occurred by TB which is different at the time of diagnosis.
The role of Mantoux test has been debatable but its positivity may not be given too much of a credence in isolation. Some studies have shown the Mantoux test to have a sensitivity of only 55% and a specificity of 80% in women with laparoscopically diagnosed TB, whereas Khanna et al. their study, found Mantoux test to be 61.5% sensitive and 66.21% specific in diagnosing TB-PCR-positive cases.[22,26] But the greater value of these tests lies in their negative predictive values. Khanna et al. in their study also showed high negative predictive value (>80%) of ESR, Mantoux test, TB enzyme-linked immunosorbent assay (ELISA), HSG and laparohysteroscopy in diagnosing GTB.[22] Therefore, if a patient is negative for all these tests, she should not be continued on anti-tubercular therapy thus avoiding the unnecessary adverse effects of anti-tubercular drugs. In the present study too, the Mantoux test and ESR were not taken in isolation but part of multiple markers of GTB and backed the imaging modalities for deciding the treatment. In concordance with Khanna et al., our study too established that in cases of GTB, the use of expensive endometrial TB-PCR tests may be avoided with a detailed work-up, which would also help in the institution of ATT in early disease, thus enhancing the chance of pregnancy.
CONCLUSION
As GTB poses a great diagnostic challenge because of its varied clinical presentations, diverse imaging pictures, and a mixed bag of bacteriological and serological tests with its own limitations, it is thus prudent not to advice the whole gamut of assays and tests, but to resort to a diagnostic modality for this disease that is simple, feasible, specific, and sensitive, and facilitates early diagnosis. Institution of ATT should also be done in early disease if these tests points to the diagnosis, so that the chances of pregnancy can be enhanced and irreversible damage to the genital organs can be prevented.
Source of Support:
Nil
Conflict of Interest:
None declared.
REFERENCES
- Detection of female genital tuberculosis by using endo-ovarian tissue biopsy. Octa J Biosci. 2013;1:98-107.
- [Google Scholar]
- Genital tuberculosis and its consequences on subsequent fertility. J Obstet Gynecol India. 2005;55:534-7.
- [Google Scholar]
- Clinical tuberculosis (1). London: Macmillan; 1992. p. :502-10.
- Female genital tuberculosis: A global review. Int J Fertil Womens Med. 2004;49:123-36.
- [Google Scholar]
- Laparoscopic observations of pelvic organs in pulmonary tuberculosis. Int J Gynaecol Obstet. 1990;32:129-31.
- [Google Scholar]
- An algorithmic approach to female genital tuberculosis causing infertility. Int J Tuberc Lung Dis. 2006;10:1045-50.
- [Google Scholar]
- Evaluation of women with infertility and genital tuberculosis. J Obstet Gynecol India. 2006;56:423-6.
- [Google Scholar]
- Human granuloma in vitro model, for TB dormancy and resuscitation. PLoS One. 2013;8:e53657.
- [Google Scholar]
- Diagnosis of culture-negative female genital tract tuberculosis with involvement of polymerase chain reaction. J Reprod Med. 2001;46:929-32.
- [Google Scholar]
- Improved diagnostic value of PCR in the diagnosis of female genital tuberculosis leading to infertility. J Med Microbiol. 2005;54:927-31.
- [Google Scholar]
- Evaluating PCR, culture and histopathology in the diagnosis of female genital tuberculosis. Indian J Med Res. 2011;134:40-6.
- [Google Scholar]
- Implantation failure: Molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242-53.
- [Google Scholar]
- Results of in vitro fertilization and embryo transfer in women with infertility due to genital tuberculosis. Fertil Steril. 1996;65:367-370.
- [Google Scholar]
- Antigonadotrophic effect of Mycobacterium tuberculosis. Horm Metab Res. 1997;29:501-3.
- [Google Scholar]
- Genital tuberculosis and implantation in assisted reproduction. Rev Gynaecol Pract. 2003;3:160-4.
- [Google Scholar]
- Comparative analysis of endometrial blood flow on the day of hCG by 2D Doppler in two groups of women with or without genital tuberculosis undergoing IVF-ET in a developing country. Arch Gynecol Obstet. 2011;283:115-20.
- [Google Scholar]
- Genital tuberculosis--a major pelvic factor causing infertility in Indian women. Fertil Steril. 1997;67:497-500.
- [Google Scholar]
- Hysterosalpingographic Appearances of Female Genital Tract Tuberculosis: Part II: Uterus. Int J Fertil Steril. 2014;8:13-20.
- [Google Scholar]
- Genital tuberculosis among infertile women and fertility outcome after antitubercular therapy. Int J Gynaecol Obstet. 2011;113:229-34.
- [Google Scholar]
- Ultrasound in female genital tuberculosis: A retrospective series. Ultrasound Obstet Gynecol. 2013;42:1-47.
- [Google Scholar]
- Diagnostic value of PCR in female genital TB and its therapeutic implications. J Obstet Gynecol India. 2009;59:67-70.
- [Google Scholar]
- Favorable infertility outcomes following anti-tubercular treatment prescribed on the sole basis of a positive polymerase chain reaction test for endometrial tuberculosis. Hum Reprod. 2012;27:1368-74.
- [Google Scholar]
- The Mantoux test in the diagnosis of genital tuberculosis in women. Int J Gynaecol Obstet. 2001;72:165-9.
- [Google Scholar]







