Translate this page into:
Comparison of metabolic parameters in hyperandrogenic and normoandrogenic women with polycystic ovarian syndrome
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
The aim was to compare the metabolic derangements in normoandrogenic and hyperandrogenic women with polycystic ovarian syndrome.
Materials and Methods:
This cross-sectional comparative study was designed to compare the metabolic derangements in normoandrogenic and hyperandrogenic women with polycystic ovary syndrome (PCOS). Hyperandrogenic women were defined on the basis of either biochemical or clinical evidence. Metabolic derangements were compared among the two groups based on various parameters including serum triglyceride level (≥150 mg/dl), high-density lipoprotein (≤50 mg/dl), fasting blood sugar (FBS) (≥100 mg%), impaired glucose tolerance (2 h postprandial BS ≥140 mg%) and fasting glucose/fasting insulin (FI) ratio (≤4.5).
Results:
Among 200 PCOS women included in this study, 120 (60%) women were hyperandrogenic whereas the rest 80 (40%) women were normal. Among the two groups, there was no significant difference between the anthropometric parameters including the body mass index, waist circumference and waist-hip ratio. Level of glucose intolerance was measured using FBS, oral glucose tolerance test and FI levels, but there was no significant difference found between the two groups (P > 0.05). Furthermore, androgen excess was not found to be associated with derangement in lipid profile.
Conclusion:
PCOS is definitely a risk factor for metabolic derangement but it is irrespective of androgen excess, as observed in the above study. Normoandrogenic PCOS women are equally at risk of metabolic derangements; therefore, they should also be screened for metabolic abnormalities at first detection.
Keywords
Hyperandrogenism
metabolic derangement
polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common and multifaceted endocrine disorder affecting 5-10% of women in reproductive age group.[1] PCOS is a major cause of oligo or anovulation leading to menstrual irregularities and subfertility. It is also associated with metabolic derangements proved by the increased prevalence of hyperinsulinemia and insulin resistance in these patients. The true pathogenesis of this heterogeneous disorder remains the topic of intense research, with most accepted etiology being genetic. The syndrome is also found to be familial with more than one gene involved in the pathogenesis, but studies have failed to find all the causative genes.[2,3]
Abbott et al. 1998[4] and Eisner et al. 2000[5] studied female rhesus monkey, exposed in utero to high levels of testosterone who later on showed biochemical and clinical features of PCOS. Later on, Homburg et al.[6] proposed that excess androgen influence the female fetus gene expression leading to PCOS phenotype that is later on exacerbated by the excess androgen production from multiple small follicles. Anovulation, presence of small antral follicles and insulin resistance in PCOS leads to increase in androgen biosynthesis that in turn forms a vicious cycle by propagating abnormal folliculogenesis. Insulin resistance, especially in obese phenotype was considered responsible for the metabolic derangements and cardiovascular morbidity, but recent studies have shifted focus to hyperandrogenemia being the "root of all evil".[7,8,9,10,11,12]
Polycystic ovary syndrome being a complex endocrine disorder presents with varied phenotypes like obese and lean PCOS. Although insulin resistance explains metabolic derangements in obese patients, but the presence of functional and structural vascular dysfunction in young, lean PCOS was found to be directly associated with high androgen index.[13] In the present study, we aimed at comparing metabolic derangements in hyperandrogenic and normoandrogenic PCOS women and establish whether androgen excess is actually the heart of this enigmatic syndrome or the mystery of PCOS needs to be solved further.
MATERIALS AND METHODS
This study was a cross-sectional observational study conducted at the University College of Medical Sciences and Guru Teg Bahadur (GTB) Hospital, a tertiary care institution in Delhi, India. A clearance from Ethical Committee of the institute was obtained and written informed consent was taken from all the subjects.
Metabolic derangements were defined as follows:
Fasting serum triglyceride (TG) ≥150 mg/dl
High-density lipoprotein (HDL) cholesterol ≤50 mg/dl
Fasting plasma glucose (FG) ≥100 mg/dl
Impaired glucose tolerance (2 h postprandial (PP) blood sugar (BS) ≥140 mg%)
Waist circumference ≥88 cm (females), waist: Hip ratio ≥0.85.
Insulin resistance in PCOS patients was defined as:
Fasting insulin (FI) ≥20 μIU/ml
FG:FI ratio ≤4.5.
Subjects
Women of reproductive age group who visited our gynecology clinic with complaints of menstrual irregularities or infertility were diagnosed as PCOS based on Rotterdam criteria, 2003.[14] Presence of two out of three of the following features was considered diagnostic of PCOS:
Oligo - or anovulation
Clinical and/or biochemical signs of hyperandrogenism (HA)
Polycystic ovaries and exclusion of other etiologies.
Women with hyperprolactenemia, thyroid dysfunction, Cushing's syndrome, acromegaly, adrenal enzymatic defects and androgen-secreting tumors were excluded from the study. Patients who were already on treatment for PCOS like those receiving ovulation induction drugs, oral hypoglycemic agents, anti-androgens or oral contraceptive pills were also excluded from the study. After taking into account, the above factors, 200 women were recruited in the study, 120 PCOS women with either clinical features or biochemical evidence of hyerandrogenism formed group I, while the remaining 80 PCOS women with no evidence of HA (NA) formed group II.
After taking written informed consent from all the study participants, a detailed history was taken, which included menstrual cycle pattern, history of weight gain, excessive hair growth along with family history of PCOS, diabetes mellitus, hypertension, coronary artery disease, and obesity. Following parameters were documented while doing general physical and systemic examination:
Height (cm)
Weight (kg)
Body mass index (BMI) calculated as weight (kg)/height (meter)2
Hip circumference (cm)
Waist circumference (cm)
Waist-hip ratio
Clinical evidence of HA-hirsutism (scored by Ferriman Gallway score and a score of >8 was considered significant), acne, androgenic alopecia
Presence of acanthosis nigricans
Blood pressure (BP).
In suspected PCOS patients a pelvic ultrasound was done on day 2 or day 3 of the cycle to confirm polycystic ovaries (presence of 10-12 follicles measuring 2-9 mm in single or both ovaries). After confirming the diagnosis of PCOS, blood samples were taken after overnight fasting on day 2 or day 3 of the cycle or on any day of the cycle in amenorrheic women for biochemical and endocrinological evaluation. Following tests were carried out using routine commercial kits in the endocrine and metabolic lab of GTB Hospital:
Serum follicle stimulating hormone, serum luteinizing hormone.
Serum thyroid stimulating hormone, free triiodothyronine, free thyroxine.
Serum prolactin.
Lipid profile: TG, HDL, low-density lipoprotein (LDL), very LDL (VLDL), HDL/LDL ratio.
Fasting serum insulin.
Fasting blood glucose and 2 h blood glucose after 75 g oral glucose load.
Total testosterone.
Comparison of metabolic parameters was done between group I (hyperandrogenic) and group II (normoandrogenic) using unpaired Student's t-test and Chi-square test. P < 0.05 was considered significant.
RESULTS
A total of 200 PCOS women were recruited in the study, among them 120 women with clinical or biochemical evidence of HA formed Group I and remaining 80 PCOS women with NA formed Group II. Majority of patients presented in the age group of 21-30 and the mean age was comparable between the two groups. Table 1 represents the comparison of anthropometric variables between hyperandrogenic and normoandrogenic PCOS women.
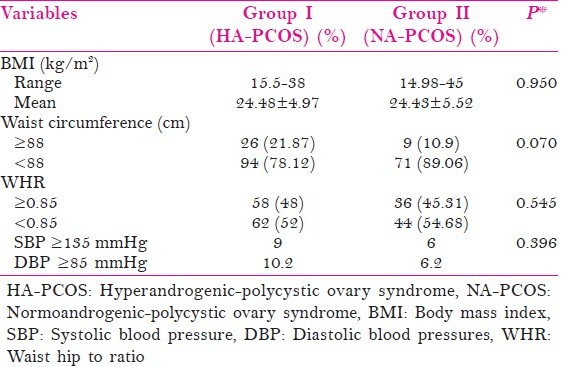
The BMI ranged from 15.5 to 45 kg/m2 with a mean of 24.48 ± 4.97 in group I and 24.43 ± 5.52 in group II, but the difference was not significant (P = 0.950). Around 22% of hyperandrogenic women had a waist circumference of ≥88 cm as against 11% of normoandrogenic women but the difference was not significant (P = 0.070). The waist hip ratio was also comparable between the two groups with 48% of hyperandrogenic women having waist to hip ratio (WHR) ≥0.85 when compared with 45% in group II (P = 0.545). Prevalence of hypertension (systolic BP [SBP] ≥135 mmHg and/or diastolic BP [DBP] ≥85 mmHg) was also comparable between the two groups that is 9% versus 6% (P = 0.396).
Table 2 shows metabolic parameters with respect to carbohydrate metabolism between HA PCOS (group I) and NA PCOS (group II) women.

Fasting blood sugar (FBS) of ≥100 mg/dl was observed in 17.7% patients in group I and 6% of patients in group II (P = 0.293). Impaired glucose tolerance (2 h PP sugar ≥140 mg%, following 75 g glucose load) was present in 9.3% patients of group I and 6.2% patients of group II (P = 0.637). Prevalence of insulin resistance was measured using FI (≥20 μIU/ml) and FG to FI ratio (≤4.5), which was again found to be comparable between the two groups [Table 2].
Fasting TG levels of ≥150 mg/dl was found in 10.4% of patients in group I and 18.75% patients in group II (P = 0.058), while HDL levels of ≤50 mg/dl was also comparable between the two groups (P = 0.277) [Table 3].

DISCUSSION
Two hundred PCOS women (120 hyperandrogenic and 80 normoandrogenic) were compared on the basis of anthropometric variables and metabolic derangements such as insulin resistance and dyslipidemia. The main purpose was to find out the effect of increased androgen index on prevalence of metabolic syndrome in PCOS women. PCOS was diagnosed on the basis of the presence of two of the three features described in the classical triad of PCOS that is, oligo or anovulation, menstrual irregularities, and ultrasonography evidence of polycystic ovaries. Hyperandrogenism was diagnosed on the basis of the presence of hirsuitism, acne and/or male pattern baldness with or without raised serum testosterone (≥1 ng/ml).
Among 120 hyperandrogenic women, 104 (86.66%) had clinical features of hyperandrogenism, but only 30 of these women also had raised serum testosterone levels, while 16 (13.33%) had raised serum testosterone levels without any clinical features. The mean BMI of hyperandrogenic PCOS (group I) was 24.48 kg/m2 which was comparable to the normoandrogenic females (group II) which was 24.43 kg/m2. These findings were in contrast to the findings of Majumdar et al.[15] who reported significantly higher prevalence of clinical hyperandrogenism (74.2% vs. 50.6%) in obese versus lean PCOS. Waist circumference of ≥88 cm was found in 21.87% of patients in group I as against 10.9% of patients in group II, but this difference was not significant. Similarly, WHR of ≥0.85 was found in 48% and 45% patients in group I and group II respectively (P > 0.05).
The incidence of hypertension (systolic BP ≥135 mm Hg and diastolic BP ≥85 mm Hg) was found to be around 10% in group I and 6% in group II, the difference was again not significant. These findings were in contrast with the findings of Chen et al.[16] who studied 151 young PCOS women and reported that high bioavailable testosterone levels (free androgen index (FAI) ≥19%) in women with PCOS increased the risk of elevated BP (SBP ≥130 mmHg and/or DBP of ≥85 mm Hg) with an odds ratio of 3.187(P = 0.029).
In our study, there was no significant difference found between normoandrogenic and hyperandrogenic PCOS women in terms of increased insulin resistance. FBS of ≥100 mg/dl, FI levels of ≥20 μIU/ml and FG:FI ratio of ≤4.5 was found in comparable number of patients in group I and group II [Table 2].
When comparison of dyslipidemia was done between the two groups, it was observed that TG levels of ≥150 mg/dl was found in 10.4% of hyperandrogenic PCOS women as against 18.75% of normoandrogenic PCOS women (P = 0.058) while HDL levels of ≤50 mg/dl was found in 78% and 67.1% females in group I and group II respectively (P = 0.277) [Table 3].
Studies have proved that anovulatory PCOS women are more insulin resistant than the weight matched controls.[17,18] Cause of this increased insulin resistance was widely believed to be due to the abnormalities in the post-receptor insulin signaling pathways and abnormal insulin secretion.[5,17,19] Abbott et al.[4] hypothesized that endocrine environment, in particular hyperandrogenemia, during development (especially during prenatal life and puberty) has profound effect on body fat distribution, thus, predisposing to insulin resistance. This hypothesis was further evaluated, and it was found that hyperandrogenemia is strongly associated with increased risk of metabolic syndrome in premenopausal PCOS women.[7,8,9] Coviello et al.[11] also found that hyperandrogenemia is a significant predictor of metabolic syndrome, independent of obesity and insulin resistance. They studied 49 adolescent girls with PCOS and concluded that the odds of having metabolic syndrome were 3.8 times higher for every quartile increase in bioavailable testosterone after adjusting for BMI and insulin resistance. Similar findings were observed by Fruzzetti et al.[20] who studied 53 adolescents with PCOS and concluded that the number of metabolic abnormalities correlated with free, total testosterone, FAI and BMI. Nisenblat and Norman[21] in their review article stressed on the fact that despite intensive research on the underlying mechanism of PCOS, there is still insufficient evidence to confirm the hypothesis of prenatal androgen exposure being the initiating and propagating factor for PCOS.
Based on the findings of the present study we hereby support the notion that normoandrogenic PCOS women are also at high risk for metabolic syndrome and thus they should also be screened for metabolic abnormalities at first detection. PCOS is an enigmatic syndrome, and larger trials are called for to establish the underlying etiopathogenesis in different phenotypes, in order to establish better therapeutic approaches. We conclude that polycystic ovarian syndrome is a risk factor for metabolic derangements per se, but it is irrespective of androgen excess. Hence, early surveillance in normoandrogenic PCOS women also, should be practiced, which will enable timely intervention in the form of lifestyle modifications or pharmacotherapy, in order to prevent long-term complications.
Source of Support:
Nil
Conflict of Interest:
None declared.
REFERENCES
- Thirty-seven candidate genes for polycystic ovary syndrome: Strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96:8573-8.
- [Google Scholar]
- Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62-7.
- [Google Scholar]
- Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206-10.
- [Google Scholar]
- The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2699-705.
- [Google Scholar]
- The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: A controlled community-based study. Fertil Steril. 2003;79:1327-34.
- [Google Scholar]
- Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929-35.
- [Google Scholar]
- Cardiometabolic risk in polycystic ovary syndrome: A comparison of different approaches to defining the metabolic syndrome. Hum Reprod. 2008;23:2352-8.
- [Google Scholar]
- Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492-7.
- [Google Scholar]
- Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: Results from the National Institutes of Health - National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276-84.
- [Google Scholar]
- Obesity is the major determinant of the abnormalities in blood pressure found in young women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2141-8.
- [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- [Google Scholar]
- Comparison of clinical features and health manifestations in lean vs. obese Indian women with polycystic ovarian syndrome. J Hum Reprod Sci. 2009;2:12-7.
- [Google Scholar]
- Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442-7.
- [Google Scholar]
- Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774-800.
- [Google Scholar]
- Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): Comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682-8.
- [Google Scholar]
- Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:2586-93.
- [Google Scholar]
- Hyperandrogenemia influences the prevalence of the metabolic syndrome abnormalities in adolescents with the polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:335-43.
- [Google Scholar]
- Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16:224-31.
- [Google Scholar]







