Translate this page into:
In Vitro Maturation
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In vitro maturation (IVM) of the human oocytes has recently found an important niche among assisted reproductive techniques (ART). Even though the ovarian stimulation protocols continue to evolve and last for few days, they are still not patient-friendly. The development of several follicles is associated with a high risk of ovarian hyperstimulation syndrome (OHSS), leading to hospitalization that can be fatal. Natural IVF cycles, mild stimulation with low dose gonadotrophins and IVM of human oocytes ready for fertilisation now offer an alternative to the traditional IVF treatment. Infertile women with polycystic ovaries or polycystic ovarian syndrome (PCOS) form the main category of patients who would benefit from IVM. However, concern exists that IVM may interfere at the epigenetic level and in particular with genomic imprinting. For normal embryonic development, timely acquisition of correct imprinting patterns in oocytes and maintenance of genomic imprinting after fertilisation is required. It is therefore necessary that patients undergoing IVM be offered preimplantation genetics screening (PGS) prior to embryo transfers. This review considers our current understanding of in-vitro maturation of human oocytes and its importance in clinical applications.
Keywords
Culture
cytoplasmic
ICSI
In vitro maturation (IVM)
imprinting
IVF
maturation
nuclear
oocyte
INTRODUCTION
Oocyte in vitro maturation (IVM) has recently found an important niche among assisted reproductive techniques (ART). It remains an enigmatic process and refers to the maturation in culture of immature oocytes after their recovery from follicles that may or may not have been exposed to exogenous follicle-stimulating hormone (FSH) but were not exposed to either exogenous luteinizing hormone (LH) or human chorionic gonadotropin (hCG) prior to retrieval to induce meiotic resumption. This process of maturation spans the time from when messages initiate germinal vesicle breakdown (GVBD) to completion of the nuclear changes resulting in expulsion of the first polar body demonstrating their readiness for fertilisation and early embryo development in vitro. The process of maturation encompasses a complex series of molecular and structural events, culminating in the arrest of the oocyte chromosomes on the metaphase II plate in anticipation of sperm penetration and activation for fertilization. Furthermore, completion of the nuclear changes required to produce a metaphase II oocyte does not confirm developmental competence and is not a reflection of the molecular and structural maturity of an oocyte, sometimes referred as cytoplasmic maturation. This technique, now offers a well-defined infertility patient groups, use of reduced amount of hormones (mild stimulation) and fertility preservation.[1,2]
It was Pincus and Enzmann (1935),[3] who first reported that immature rabbit oocytes matured spontaneously in vitro over 12 hours after being aspirated from their follicles into culture media. Edwards (1965),[4] concurred with the timings as being ~12 hours in mice, rats, and rabbits, whereas oocytes from cattle, sheep, rhesus monkey, and humans required longer intervals in vitro, e. g., ~31 hours in cows and rhesus monkeys, ~37 hours in humans, and 40 hours in pigs. Maturing oocytes in vitro was simple, required no hormones and opened pathways to studies on oocyte maturation and fertilization in vitro. Moreover, the timings for full maturation in vivo to metaphase II after an injection of hCG in various species were found to be identical with the timing of oocyte maturation in vitro (Edwards, 1965).[4]
However, it was not until 1968, when Edwards and colleagues[5] confirmed that human follicular oocytes were also able to mature in vitro when isolated from follicles and placed in appropriate culture medium. Subsequently, the first successful human pregnancy from in vitro fertilization (IVF) of the oocytes retrieved from natural ovulatory cycle was achieved in 1978.[6] More than 2,000 children have been born after the successful fertilization, development, and pregnancy with immature human oocytes matured in vitro.[7,8,9,10,11,12,13,14,15,16,17,18,19] Although the results of the children's follow-up are generally reassuring,[20] there remains a concern about the possible interference of these techniques with epigenetic mechanisms and, in particular, with genomic imprinting.[21]
Even though the ovarian stimulation protocols continue to evolve and last for few days, they are still not patient-friendly. The development of several follicles is associated with a high risk of ovarian hyperstimulation syndrome (OHSS), leading to hospitalization that can be fatal.[19] There are issues such as the long-term effects of IVF on children born and the long-term implications on a woman's health that still need to be addressed.[22] Ultimately, the low implantation rate after transfer of morphologically high quality embryos has led to the hypothesis of decreased endometrial receptivity, resulting from the exposure of the endometrium to supraphysiological steroid hormones during ovarian stimulation.[23]
Infertile women with polycystic ovaries or polycystic ovarian syndrome (PCOS) form the main category of patients who would benefit from IVM.[24] In this group of patients, nearly 50% of immature oocytes reach their maturation after 24-28 hours with nearly 20-25% clinical pregnancy.[25] However, this group only represents 20-30% of infertile patients. It has been reported that normovulatory women, irrespective of the infertility cause have similar results to PCOS group.[18] Therefore, more recently, IVM has been extended to the normovulatory, the poor responders and to those women interested in natural cycles. Moreover, IVM in natural cycle can be considered as a social and economic alternative to classical ART on the basis of cost-effectiveness. Furthermore, successful cryopreservation of immature oocytes would enlarge the target treatment group and offer the potential to store oocytes either for patients at risk of loosing their fertility capability (cancer survivors), or in countries where ethical and legal problems have been raised.[1,16,26]
One other area that may benefit from IVM program and needs exploring is stem cell technology for cell replacement therapies. Currently, due to lack of viable spare embryos, restrictions from regulatory bodies in some countries and opposition from various religious groups, establishment of new human stem cell lines have been very slow. Through IVM program, it is feasible to overcome many of the social and ethical restrictions.
Although there has been remarkable progress made both in laboratory techniques and composition of culture media and conditions, the clinical management of IVM cycles have lagged behind. There is an urgent need to develop not only clinical protocols but also well-defined culture medium and conditions that would support oocyte maturation.[16] It is important to understand that inter- and intra-cellular mechanisms and how oocyte-derived factors influence zygotic genome activation and embryonic development competence.[16,19]
This review addresses our current understanding of IVM of human oocytes and its importance in clinical applications.
OOCYTE GROWTH AND MATURATION
Female germ cells enter meiosis early in foetal life, and a finite number of oocytes are determined before birth. The first meiotic germ cells appear around week 11, and shortly before birth there are in total 1-2 million oocytes arrested in prophase I (PI) of first meiotic division and continue to be arrested in this phase during growth [Figure 2] a.[27]
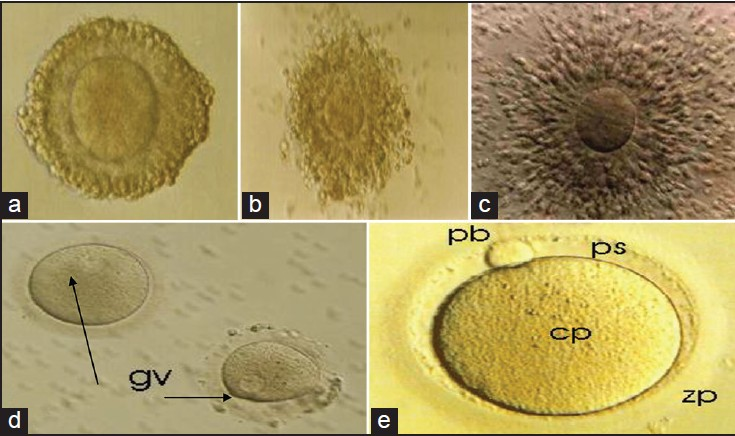
- (a) Immature oocyte retrieved without human chorionic gonadothropin (hCG) priming showing compact cell layers surrounding the oocyte: Prophase I. This oocyte probably contains an intact germinal vesicle, although it cannot be seen in this micrograph. Incubation for prolonged time period (>24 hours) under in vitro fertilization (IVF) condition and i in vitro maturation (IVM)® Medium (CS; Cat. No: 8221) resulted in oocyte maturation, (b) Oocyte maturation after 20 hours of culture in in vitro maturation (IVM) medium. Note the dispersion of cumulus cells surrounding the oocyte, (c) Matured oocyte with fully expanded cumulus oophorus and corona radiata after 37 hours, (d) Denuded immature oocyte with visible germinal vesicles (gv), (e) Denuded metaphase II oocyte, showing a small perivitelline space (ps), a clear moderately granular cytoplasm (cp), an ovoid or round first polar body with smooth surface (pb), and a clear colorless zonapellucida (zp)
It has been well-documented that no more than 400 follicles (oocytes) reach ovulation during a woman's reproductive life.[27] The great majority of follicles (99%) get destroyed through atresia. Normally, the growth phase of the primordial follicles is characterized by an increase in size of the oocyte from a diameter of 30 mm to a final diameter of 120 mm, and by proliferation of granulosa cell and theca cell differentiation. Furthermore, there is a significant increase in cytoplasmic organelles besides the appearance of new structures such as the cortical granules. Active transcription and translation during growth allows accumulating stores of proteins essential for later stages of oocyte maturation and are required to support the early embryonic cell divisions.[27]
Moreover, the oocyte secretes glycoproteins, which condense around it to form a translucent cellular layer called the zona pellucida. The zona separates the oocyte from the surrounding granulosa cells. However, these cells maintain their contact with the oocyte via cytoplasmic processes, which penetrate the zona to form gap junctions at the oocyte surface. Gap junctions also provide the basis for an extensive network of intracellular communication among granulosa cells.
Transcription ceases at the time of ovulation, and therefore the oocyte and pre-embryo are dependent on the maternally inherited reserves of ribonucleic acid (RNA) and protein until the embryonic genome is activated and RNA synthesis is initiated. The commencement of embryonic transcription varies between species; in humans, this is thought to occur at the 4-6 cell stage.[27]
Nuclear maturation
Oocyte maturation which includes nuclear and cytoplasmic events has been extensively reviewed.[13,16]
Nuclear maturation (meiotic resumption and progression from PI to MII, see Figure 2a-e) occurs spontaneously when the follicular inhibitory signal is suppressed. Prior to meiotic maturation, the oocyte nucleus is characteristically large and pale. Following resumption of meiosis, the nuclear membrane dissolves [GVBD, Figure 2d] and the homologous chromosomes become separated with extrusion of the first polar body into the perivitelline space. After completion of the first meiotic division, the second meiotic division is initiated and the oocytes reach MII prior to ovulation [Figure 2e]. More recently, it has been reported that IVM can have deleterious effects on the organization of the meiotic spindle and chromosome alignment in the human oocytes.[28] Aneuploidy resulting from errors in meiotic chromosome segregation is the leading cause of pregnancy losses.[28] This could explain the reduction found in developmental competence of IVM human oocytes compared with those matured in vivo and supports the need for improving in vitro culture conditions.
Cytoplasmic maturation
Even though oocytes may be able to achieve complete nuclear maturation, they may still be deficient in cytoplasmic maturation.[29] It is recognized that most deficiencies in oocytes during maturation are mainly associated with cytoplasmic reprogramming rather than meiotic progression. The effects of cytoplasmic aberrations are seldom expressed at an early stage of development, but instead are more frequently associated with cleavage and pre-implantation stages.[30] Furthermore, cytoplasmic maturation is indirectly and retroactively assessed as the ability of the mature oocyte to undergo normal fertilization, cleavage, and blastocyst development.[30] It is important to note that human oocytes surrounded by the cumulus cells have a higher maturation rate than those without these cells.[31] Indirect morphological parameters taken into account to evaluate cytoplasmic maturation include cumulus cell expansion, expulsion of the polar body, and an increased perivitelline space [Figure 2e].
Oocyte maturation is also influenced by complex interactions of different intracellular, paracrine, and structural factors, including sterols, steroids, growth factors, cyclic adenosine monophosphate, and the presence of gap junction.[32] Many of these factors and regulatory molecules have been shown either to inhibit or to promote nuclear and cytoplasmic oocyte maturation, directly or via the cumulus cells.[16]
A successful pregnancy is dependent on achieving satisfactory maturation. The stage at which pregnancy terminates varies depending on the nature of the error induced during maturation. It has been suggested that gross abnormalities interrupt the meiotic cycle or block fertilization. Although, subtle imperfections during maturation may be manifested during late cleavage or blastocyst stages, survival to term has been a guide to controlling acquisition of full developmental competence during maturation.[30]
CLINICAL MANAGEMENT OF IVM
Patient selection
During controlled ovarian stimulation, the oocyte population at the time of the hCG administration may be heterogeneous, leading to retrieval of oocytes at different stages of maturation. Moreover, about 15% of oocytes remain in prophase I of meiosis and can be matured in vitro to develop into viable embryos which, when transferred result in live births.[8,9,10,11] It is therefore highly probable that these oocytes represent an inferior population as they failed to mature when exposed to supra-physiological concentrations of gondotrophins.
Most of the experiences in human-IVM have been obtained from two main groups, PCOS and regular cycling women. Women suffering from PCOS are extremely sensitive to FSH and are at a significant risk of developing OHSS. The regular cycling women have normal ovaries and are referred for IVF (ICSI) due to severe male infertility. In addition, poor responders and those wishing for natural cycles may also benefit from IVM as would patients requiring fertility preservation.[1,2]
IMMATURE OOCYTES OBTAINED FROM WOMEN WITH PCOS
In 1994, the first pregnancy and delivery of a healthy baby after IVM of immature oocytes obtained in a patient with PCOS was reported.[9] The main "advantage" of IVM for women with PCO or PCOS is the presence of multiple antral follicles that are easily assessed by transvaginal ultrasonography. Given the high risk for OHSS during multifollicular stimulation for IVF, it was recognized that this subgroup of infertility patients may benefit from IVM.[9] It has been established that the developmental capability of primary oocytes is higher in regular cycling women than in irregularly cycling and anovulatory women with PCO.[10] To compensate for this observation, endogenous priming with FSH.[18,31] or hCG.[16] has been suggested before oocyte retrieval and IVM.
Use of low-dose (37.5 IU) recombinant FSH for 3 days followed by deprivation for 2-3 days before aspiration until the leading follicle reached 10 mm has resulted in maturation and fertilization rates in women with PCOS comparable with those in regularly cycling women.[31] Moreover, FSH priming improved the pregnancy (29 versus 0%) and implantation (21.6 versus 0%) rates compared with the non-primed group.[31] Both FSH priming and subsequent FSH deprivation caused by withholding exogenous FSH should enhance the competence of the oocytes. It has been reported that oocyte differentiation may be incomplete during follicular growth, and that oocytes from plateau phase follicles have increased competence.[10]
Additional FSH priming (75 IU per day for 6 days initiated on day 3) did not influence the oocyte recovery, the maturational and the developmental potential, the fertilization rate, or the pregnancy rate.[10] Administration of 10,000 IU hCG, 36 hours before oocyte retrieval not only improved maturation rate of immature oocytes from PCOS women, but also hastens the maturation process.[16] Pregnancy rates of 30-35% have been obtained in a multi-center study, including cycles with hCG priming before immature oocyte retrieval.[16] The potential mechanism of the action of hCG on these small follicles is unclear.
IMMATURE OOCYTES FROM REGULAR CYCLING WOMEN WITH NORMAL OVARIES
The first birth reported from IVM of immature oocytes from unstimulated cycles used oocytes that had been retrieved at different times during the menstrual cycle.[10,12]
Control of the menstrual cycle is a complex process involving both the hypothalamic-pituitary axis and local (paracrine and endocrine) factors.[31] It is recognized that circulating concentrations of FSH and LH regulate follicular growth and development. Furthermore, synthesis of oestradiol is closely linked to the development of the preovulatory follicle and the size of the follicle is indicative of the concentration of oestradiol in the follicle and serum. The increase in the concentration of oestradiol is the principal factor for establishment of dominance. Physiologically, Oestradiol has a negative feedback influence on the hypothalamic axis with subsequent decrease in the concentration of FSH. The dominant follicle withstands this decline, while subordinate follicles are susceptible to a decline in gonadotrophins and undergo atresia. The subordinate follicles, however, can be rescued and thereby avoid atresia by retrieval of immature oocyte and IVM. It has been reported that timed oocyte collection to coincide with selection of the dominant follicle resulted in 18% pregnancy rate.[18]
Immature oocytes from poor responders
Poor response to ovarian stimulation by gonadotropins is defined as a plasma oestradiol level of <1000 pg/ml on the day of hCG injection and retrieval of less than four mature oocytes.[33] Moreover, following gonadotrophin stimulation, the number of follicles in this group of patients may be normal, but their size are smaller than in the usual treatment cycles. Despite this observation, a pregnancy rate of 37.5 % (three out of eight) has been reported following immature oocyte retrieval and IVM without hCG administration before oocyte collection.[34] However, administering of 10,000 IU hCG, 36 hours before oocyte retrieval followed by IVM, optimized the successful pregnancy rate as it allows collecting some in vivo matured oocytes. When these mature oocytes are pooled with IVM of immature oocytes, it was possible to maximize the successful IVF treatment without cycle cancellation.[33]
Immature oocytes from natural cycle IVF
In recent years, interest in natural cycle IVF among patients has been encouraged because of IVM. Primarily the treatment is more comfortable, there are less side effects, and the only disadvantage is failed oocyte collection and as a consequence possibility of no embryo for transfer. As discussed earlier, although only a single follicle usually grows to the pre-ovulatory stage and releases its oocyte, there are many small follicles that also develop during the same follicular phase of the menstrual cycle. It is believed that approximately 20 antral follicles are selected and continue to the pre-ovulatory stages of development during each cycle.[32] It has been suggested that atresia does not occur in non-dominant follicles even after the dominant follicle has been selected during folliculogenesis.[14] Thornton and colleagues.[14] retrieved immature oocytes from non-dominant follicles and successfully matured them in vitro, fertilized and reported several healthy live births.[14] This procedure, therefore, offers a very attractive possibility for a success natural cycle IVF when combined with immature oocyte retrieval and IVM. However, care should also be taken to ensure that no ovulation from the dominant follicle occurs due to an endogenous circulating luteinizing hormone surge. In natural cycle IVF patients combined with IVM, it is recommended that a 10,000 IU hCG be administered 36 hours before oocyte retrieval when the size of the dominant follicle reaches 14 mm in diameter.
PATIENTS AND MONITORING
All our patients undergoing IVM-ICSI cycle have a transvaginal ultrasonography on day 3 of the menstrual cycle. This is to confirm the absence of ovarian cysts and to evaluate endometrial thickness. Patients with ovarian cysts >11 mm or endometrial thickness >6 mm and oestradiol serum concentration >50 pg/ml have their cycles cancelled. On the same day, base line measurements of FSH, LH, and Oestradiol areobtained. Hormone profiles are monitored from day 3 until the day of oocyte retrieval and the follicular diameter is calculated as the mean of the longest follicular axis and the axis perpendicular to it in the same scanning plane.
No FSH stimulation is used prior to the retrieval of immature oocytes for patient with regular menstrual cycles. PCOS patients are always primed with r-FSH 150 IU/day for 3 days, starting on day 3 of the menstrual cycle.
To ensure follicular growth and to exclude any development of a dominant follicle >13 mm, an ultrasound scan is repeated between days 6 and 8 of the cycle. Oocyte retrieval is scheduled within 24 hours, once a leading follicle of 9-11 mm in diameter and an endometrium thickness of ≥6 mm are seen on transvaginal ultrasonography. hCG priming is given prior to oocyte retrieval as reported elsewhere.[18]
Normally, the cycle is cancelled if any patient has a dominant follicle >13 mm and endometrial thickness of <4 mm.
ENDOMETRIAL PREPARATION
It has been observed that puncturing follicles before they have reached maturity have resulted in an endometrium that was inadequately primed for implantation, possibly due to lack of adequate endogenous oestradiol and progesterone produced by granulosa cells.[9] Therefore, in order to synchronize the window of implantation with embryo development, exogenous priming with estradiol and progesterone is essential. It is well-known from hormone replacement in recipients of donor oocytes that 2-day-old embryos are best transferred into the uterine cavity on days 3 or 4 of progesterone exposure.[35]
Furthermore, it has been reported that mid-follicular endometrial priming initiated with 1-2 mg/day of 17 β-oestradiol between cycle days 5 and 7, and gradually increased by 1-2 mg/day until oocyte retrieval resulted in more successful maturation (60%), fertilization (75%), and cleavage (92%) when compared with the early priming initiated on cycle day 3.[12]
Barnes and colleagues[10] have proposed that by luteinization of the dominant follicle with hCG priming at the time of immature oocyte recovery, embryo and uterine synchrony were potentially enhanced, which resulted in better pregnancy outcome. Although, pre-retrieval hCG priming has been shown to increase the pregnancy rate in IVM, a randomized study has eluded to the fact that this may possibly be due to more embryos being generated rather than improvement of endometrial receptivity documented by ultrasound parameters such as endometrial thickness, uterine artery pulsatility index, and sub-endometrial blood flow.[36]
In most of the current protocols, 6-10 mg/day of oestradiol is started on the day of oocyte retrieval and the dose is adjusted based on the thickness of endometrium. On the day of fertilization of the matured oocytes (mostly with ICSI), progesterone is administered along with oestradiol preferentially transvaginally (200 mg, three times a day) and continued until 12 weeks.[18]
OOCYTE RETRIEVAL
Although, the importance of the timing of oocyte retrieval in human IVM is unclear, it has been reported that fewer number of oocytes with decreased maturation and fertilization rate are retrieved in the follicular phase when the dominant follicle has exceeded 14 mm in diameter.r[13] Current practice, however, suggests oocyte retrieval timing to be when the follicular range is between 8-12 mm. The possibility exists that criteria for follicle aspiration in PCOS patients should be different from those for normo-ovulatory patients.
ASPIRATION TECHNOLOGY
The retrieval procedure of immature oocytes is no different from that used for the retrieval of mature oocytes from stimulated follicles. The only difference being that the follicle diameter is much smaller and visualization requires closer familiarity with such images. However, recent improvements in ultrasound technology (3D) have assisted the operator and lead to a higher oocyte retrieval rate even from smaller diameter follicles.
Type of anesthesia
In previous studies, transvaginal oocyte aspiration has been performed under general anesthesia,[37] spinal anesthesia,[16,38] or using para-cervical block and conscious sedation, which is well-accepted by the patients.[12,18] However, it has been reported that Propofol is detrimental to mouse embryos and accumulates in the follicular fluid, with increasing concentration throughout the procedure.[34] As there are no studies so far to demonstrate that Propofol has any detrimental effect on human IVM, and as similar results regarding the maturation, fertilization, and pregnancy rates have been achieved compared with previous studies using different anesthesia methods,[34,38] centers continue to use the anesthetic regime best suited for the patient.
Aspiration pressure
In an animal model, it was observed that increasing the aspiration pressure tended to reduce the portion of cumulus-oophorus-complex (COC) with intact cumulus, whereas a higher aspiration pressure provoked an increase in the number of denuded oocytes. These results demonstrate that variation in aspiration pressure affects oocyte recovery rate and COC quality.[39] In addition, it has also been shown that reducing the size of the needle used for oocyte collection from 15- to 17- or 18-gauge reduces pain without affecting the number of oocytes collected, their quality, or the clinical pregnancy rate.[40] In our clinical setting, a 17-gauge double lumen needle and decreased aspiration pressure of 80 mmHg is used. Most reports describe the use of a single lumen needle under ultrasound guidance, and follicles of 2-10 mm may be aspirated usually with 2-3 flushes. However, in agreement with other groups who do not flush, there appears to be no proven benefit of flushing.[10,12,15,16] Finally, our experience has shown that mild curettage of follicles (mainly 6-12 mm) could mechanically increase the chance of aspirating an oocyte.
IVM AND CULTURE CONDITIONS
Although numerous data have been accumulated from animal studies, very few reports based on human data are available on the composition of culture media for human oocyte maturation. One of the main reasons has been that too few GV oocytes have been available to make meaningful comparison.[41] Even though different culture media have been used to mature human oocyte, no one medium has been shown to be clearly superior. The current rationale for choosing a specific medium for IVM of immature oocytes appears to stem largely from adapting methods developed from culturing non-ovarian somatic cells. Given the apparent need to test the different IVM base media in different species, the choice of base medium for human IVM is particularly difficult. In human IVM, tissue culture medium 199 (TCM-199) has most often been used as a maturation medium. Different groups working in this area have added varying concentration of synthetic serum substitute (SSS), FSH, LH, estradiol, insulin, human transferrin (TF) fibroblast growth factor (FGF), and epidermal growth factor (EGF), to TCM-199.
Other media that have been used include, Waymouth MB 752/1, Ham's F-12, Minimum Essential Medium (MEM), Dulbeceo's Modification of Eagle's Medium (DMEM). Hamster Embryo Culture Medium (HECM) and Chang's medium buffered with bicarbonate or N-2 hydroxyethylperazine N-2-ethane sulphonic acid (HEPES) [Table 1]. Although major beneficial components are present in all these media,[42] it is necessary to determine specific metabolic needs and optimal culture conditions required by maturing oocytes for appropriate gene expression and regulation. Recently, reports have documented the occurrence of specific cell cycle modification in vitro matured oocytes including accelerated meiotic progression and premature formation of cytoplasmic microtubules. It is possible that this could lead to mitotic spindle defectiveness that does not impair oocyte maturation and fertilization, but results in arrest of development and generation of embryos with multinucleation or chromosomal abnormalities.

No significant increase in maturation rates has been reported when follicle-stimulating hormone (FSH) was added to the maturation medium. However, in women undergoing oocyte retrieval during stimulated cycles, significantly higher FSH concentrations have been reported in the fluid of follicles containing metaphase I (MI) and metaphase II (MII) oocytes, compared with those containing germinal vesicle (GV) stage oocytes. FSH is necessary for the induction of luteinizing hormone (LH) receptors in pre-ovulatory follicles. Furthermore, addition of LH to culture medium induces GVBA in cultured intact follicles. Although it is conventionally believed that there are no LH receptors on the oocytes, recent reports have shown that messenger ribonucleic acid (mRNA) for FSH and LH receptors are present in mouse oocytes, zygotes, and perimplantation embryos, indicating a potential role for the gonadotropins in the modulation of meiotic resumption and completion of oocyte maturation.[13] It is therefore necessary to carefully evaluate and understand the mechanism of gonadotrophin actions on oocyte maturation.
Over the last few years several human oocyte in vitro maturation (IVM) media have been made available commercially. As none of the commercial companies are willing to disclose the composition of their IVM media, it is difficult to make a meaningful assessment of safety and efficiency of these IVM media to mature human oocytes. Figure 1, adopted from Chian and colleagues.[16] Illustrates the possible dialogue between participating factors involved in oocyte maturation during culture in vitro.

- Hypothetical model for the possible participation of factors in human oocyte maturation during culture in vitro. Abbreviations: FSH = follicle-stimulating hormone, LH = Luteinizing hormone, FSHR = FSH receptor, LHR = LH receptor, GV = Germinal vesicle, E2 = Estradiol, P4 = Progesterone, ER = Estradiol receptor, PR = Progesterone receptor (Adopted from Chian et al., 2004)[16]
TIME INTERVAL OF MATURATION
Previous studies have shown that 80% of immature human oocytes show nuclear maturation (extrusion of a polar body) and will be at MII by 48-54 hours of culture.[9,12] However, significant asynchrony of maturation has been observed and a number of MII oocytes have been obtained after 24 hours of maturation. Had these oocytes been inseminated after 48 hours, they would have been at MII arrest for 20-30 hours, which would place them well past the optimal fertilization time and possibly compromise their developmental competence. Adverse consequences have also been observed when aged oocytes that were already at MII by 23-25 hours were inseminated late.[10] Although, the optimal time of insemination has not yet been established, 28 hours IVM period has a significant benefit in that it allows insemination to be performed during working hours; otherwise it would have to be performed at night when the 36 hours IVM schedule is used.
LABORATORY PROTOCOL FOR IVM CULTURE
Laboratory protocols for IVM procedures are highly dependent on the type of media used. In my laboratory, we use IVM® medium from copper surgical (CS). Prior to oocyte collection, 3 ml LAG medium (Vial 1, CS; Cat No. 8201) and 10 ml of IVM® medium (Vial 2, CS; Cat no. 8220) is pre-equilibrated in a 5-6% CO 2 environment at 37°C for a minimum of 12 hours.
The follicular aspirates are transferred in tubes to the laboratory and washed on either a cell strainer with 70 μm of pore size (no. 352350; Becton Dickinson Falcon, USA), or an embryo filter (Falcon 1060) also with a pore size of 70 μm. This washing step serves to remove erythrocytes and small cellular debris and helps with the identification of COC. The retained cells are than re-suspended in flushing medium with 10 IU/ml of heparin (CS: Cat. No: 1076) maintained at 37°C. After examination and classification, the identified COC are pooled together and washed twice with the same flushing medium. They are divided into groups of three and placed in a 4-well dish containing 0.5 ml of pre-equilibrated Lag Medium (vial 1 of IVM® system medium;) under paraffin oil and incubated at 37°C and 5-6% CO 2, humidified atmosphere for 3 hours. After 3 hours, each group of oocytes is transferred into a new pre-equilibrated 4-well culture dish with 0.5 ml of IVM Medium (vial 2 of IVM® system medium) under paraffin oil, supplemented with recombinant FSH 0.075 IU/ml (Merck Serono), hCG 0.5 IU/ml (Merck Serono), and 10% heat inactivated (56°C) maternal serum, normally obtained on the same day as the oocyte collection. The heat inactivate serum is filtered through a double 0.22 μm millipore filter prior to use. The oocytes are incubated for a further 26 hours at 37°C and 5-6% CO 2 humidified atmosphere. The maturity of oocytes is determined under stereomicroscope at each change over. The oocytes with expanded cumulus cells are than cultured separately in 30 μl drops of Universal IVM medium with Phenol Red (CS: Cat No:1031/1030) under paraffin oil, pre-equilibrated at 37 ° C, until needed for injection [Figure 2c].
Prior to injection, oocytes with expanded cumulus cells are denuded with mechanical pipetting. Oocytes are briefly exposed to hyaluronidase solution 80 IU (hyaluronidase 80 IU/ml; Sage Media, USA). Oocytes are classified as having undergone germinal vesicle breakdown when the nuclear membrane is absent and as a mature MII oocyte when the first polar body has been extruded [Figure 2e].
For ICSI, denuded oocytes are placed individually into 5 μl drops of sperm preparation medium with Phenol Red (CS: Cat. No: 1070) and 2 μl of prepared sperm suspension is added to a 5 μl drop of PVP clinical grade (CS; Cat. No: 1090). A single spermatozoon is injected into each MII oocyte. After ICSI, each oocyte is transferred into a 30 μl droplet of Universal IVF medium as mentioned above. Fertilization is assessed 16-18 hours after injection for the presence of two distinct pronuclei and two polar bodies. All the resulting pre-zygotes are individually cultured in pre-equilibrated micro-drops of 15 μl of ISM1 medium (CS; Cat. No: 1050/1150) under liquid paraffin (CS; Cat No.1010). The embryos are cultured for 2 days and are evaluated daily by observing the percentage of fragmentation and the number of regular size blastomeres. Before transfer, all embryos are pooled and scored on a scale of 1-4, where type 1 and 2 (<10% fragments) are considered to be transferable.[43] A maximum of two embryos are normally transferred after discussion with the patient. All the oocyte handling is carried out on heated stages at 37°C.
The semen samples are normally collected at 14:00 hours on the day after oocyte retrieval. Motile sperms are collected by processing the liquefied semen sample on discontinuous gradients (47.5 and 90%) of Sil-Select (FertiPro, Beernem, Belgium). The pellet containing motile spermatozoa is washed and suspended in Universal IVF Medium (CS; Cat No: 1031/1030) and stored in an incubator at 37°C and 5-6% CO 2 humidified atmosphere until required.
Any grade 1 or 2 embryos remaining after the embryo transfer are routinely frozen using a slow freezing protocol. Recently, we have introduced vitrification as part of our freezing program and all future spare embryos will be divided into slow-freezing and vitrification groups to assess the survival of IVM-embryos using these two techniques. The embryo development rate is defined as the number of transferable embryos out of the total number of oocytes injected and the implantation rate as the number of gestational sacs seen on ultrasound examination out of the total number of embryos replaced.
CONCLUSION
IVM treatment has gained popularity and more than 2,000 healthy live births have been reported all over the world following immature oocyte retrieval and IVM. This procedure has seen a decrease in OHSS by eliminating or minimizing the use of gonadotrophins in women with PCOS. Although, detailed analysis of the follow-up of these babies is awaited, a growing number of fertility centers currently using the IVM technique for assisted reproduction consider it an effective and safe.[16,18,31,38,44] The high percentage of good quality embryos confirms that in vitro matured oocytes have the same possibility as in vivo matured oocytes to progress in normal cleavage and development.
Furthermore, studies have shown that IVF with GnRH-antagonist protocol is associated with lower incidence of OHSS. The number of oocytes retrieved was higher in the IVM group, whereas the number of mature oocytes, fertilization rate, and number of embryos cleaved were comparable. Although the implantation rate was higher in the IVF group, clinical pregnancy rates per embryo transfer were not statistically different (IVF: 45.8% versus IVM: 32.4%). It is worth noting that the live-birth rate was higher in the IVF group (IVF: 40.7% versus IVM: 23.5%; P = 0.04)[45] None of the women in the IVM group developed OHSS compared to five in the IVF group.
Behavior of cytoplasmic organelles and cytoskeleton during oocyte maturation has been reviewed by Mao and colleagues.[46] It appears that the cytoplasm of the oocyte is of key interest in oocyte maturation. Furthermore, supplementation of gonadotrophins (FSH, LH) to the maturation medium has benefited the process of cytoplasmic maturation of immature human oocytes.[13,29] Although immature oocytes can be maintained in culture for approximately 28-32 hours prior to the insemination procedure, it is also necessary to note that the culture in specific medium induces the resumption of meiosis until the stage of MII and allows the onset of cytoplasmic competence.[46] The high maturation rate observed confirms that the specific medium is able to synchronize the process of cytoplasmic and nuclear maturation within 30 hours of culture.
In vitro follicle culture in combination with IVM may become possible in future as progress is currently being made toward complete development of follicular oocytes in vitro.[47] So far, complete in vitro follicle growth from primordial follicle up to Graafian stage has been achieved only in mice.[29] Methods for long-term in vitro follicle growth of human primordial follicles are at present under development,[48] but a successful culture system has not yet been reported. It is encouraging that human primordial follicles have been found to proliferate up to secondary follicles even after freeze-storage,[49] allowing a greater scope for IVM procedures.
Current research in optimizing methods for the freezing of isolated immature oocytes and of a complete human ovary will benefit from IVM. This may have advantages in assisted reproductive technologies and will help to restore fertility in the treatment of cancer in children and young women.[26]
Furthermore, in this group of patients monitoring is simple, both for patients and clinicians as gonadotrophins are not administered and so the costs and long-term side effects are reduced.
Although, there have been no alarming reports on the safety of the IVM procedure or on the normality of the babies born so far, clinic offering IVM should consider preimplantation genetic screening and other antenatal genetic evaluation as an integral part of their IVM program and reduce any genetic abnormalities in the IVM babies.[50,51,52]
As with the advent of IVF or ICSI, improving clinical management in combination with appropriate culture media and techniques will help with enhancement of clinical pregnancy rates. However, further research is still needed to improve IVM technique, in order to achieve higher success rates comparable with gonadotrophin stimulated cycles. IVM might become suitable for more subgroups of infertile patients and perhaps lead to a less-invasive assisted reproduction treatment, accessible to a larger fraction of the infertile population.
Source of Support:
Nil
Conflict of Interest:
None declared.
REFERENCES
- Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: Implications for fertility preservation. Hum Reprod Update. 2010;16:395-414.
- [Google Scholar]
- The promise of in vitro maturation inassisted reproduction and fertility preservation. Semin Reprod Med. 2011;29:24-37.
- [Google Scholar]
- The comparative behaviour of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J Exp Med. 1935;62:655-75.
- [Google Scholar]
- Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221:632-5.
- [Google Scholar]
- Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109-13.
- [Google Scholar]
- Maturation and fertilization of morphologically immature human oocytes in program of in vitro fertilization. Fertil Steril. 1983;39:594-602.
- [Google Scholar]
- In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353-62.
- [Google Scholar]
- Production of embryos from in vitro-matured primary human oocytes. Fertil Steril. 1996;65:1151-6.
- [Google Scholar]
- Successful in vitro maturation of human oocytes not exposed to human chorionic gonadotropin during ovulation induction, resulting in pregnancy. Fertil Steril. 1997;67:566-8.
- [Google Scholar]
- Unstimulated immature oocyte retrieval: Early versus midfollicular endometrial priming. Fertil Steril. 1997;67:616-20.
- [Google Scholar]
- Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103-20.
- [Google Scholar]
- Immature oocyte retrieval: Lessons from unstimulated IVF cycles. Fertil Steril. 1998;70:647-50.
- [Google Scholar]
- Pregnancies and deliveries after in vitro maturation culture followed by in vitro fertilization and embryo transfer without stimulation in women with polycystic ovary syndrome. Fertil Steril. 2000;73:978-83.
- [Google Scholar]
- Combination of FSH priming and HCG priming for in-vitro maturation of human oocytes. Hum Reprod. 2003;18:1632-6.
- [Google Scholar]
- Strategies in human in-vitro maturation and their clinical outcome. Reprod Biomed Online. 2005;10:593-9.
- [Google Scholar]
- Immature oocytes in-vitro maturation: Clinical aspects. Reprod Biomed Online. 2005;10:587-92.
- [Google Scholar]
- Ghumman S, ed. Epigenetics and ovarian stimulation. Principles and practice of controlled ovarian stimulation in ART. Springer: Press; 2014.
- Children born after assisted reproductive technology. Am J Perinatol. 2002;19:59-65.
- [Google Scholar]
- Reproductive biology and IVF: Ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84-90.
- [Google Scholar]
- In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod Biomed Online. 2004;8:547-52.
- [Google Scholar]
- Ongoing twin pregnancy after ICSI of PESA-retrieved spermatozoa into in-vitro maturated oocytes: Case report. Hum Reprod. 2001;16:1424-6.
- [Google Scholar]
- Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68:724-6.
- [Google Scholar]
- Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr Rev. 1996;17:121-55.
- [Google Scholar]
- Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827-32.
- [Google Scholar]
- Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197-207.
- [Google Scholar]
- Factors affecting the developmental competence of mouse oocytes grown in vitro: Follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445-53.
- [Google Scholar]
- Luteal phase start of low-dose FSH priming of follicles results in an efficient recovery, maturation and fertilization of immature human oocytes. Hum Reprod. 2000;15:747-51.
- [Google Scholar]
- The role of intra-ovarian interactions in the regulation of follicle dominance. Hum Reprod Update. 1999;5:153-65.
- [Google Scholar]
- Gonadotropin-releasing hormone antagonist protocol: A novel method of ovarian stimulation in poor responders. Eur J Obstet Gynecol Reprod Biol. 2001;97:202-7.
- [Google Scholar]
- Pregnancies and birth achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil Steril. 2003;80:447-9.
- [Google Scholar]
- Endometrial preparation: Lessons from oocyte donation. Fertil Steril. 1996;66:873-84.
- [Google Scholar]
- Human chorionic gonadotropin for in vitro oocyte maturation: Does it improve the endometrium or implantation? J Reprod Med. 2004;49:93-8.
- [Google Scholar]
- Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in vitro maturation. Hum Reprod. 1998;13:3132-8.
- [Google Scholar]
- In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76:936-42.
- [Google Scholar]
- Ovum pick up in swine: The influence of aspiration vacuum pressure on oocyte recovery from preovulatory follicles. Acta Vet Hung. 1997;45:189-96.
- [Google Scholar]
- A prospective randomized study comparing needles of different diameters for transvaginal ultrasound-directed follicle aspiration. Fertil Steril. 1996;65:109-13.
- [Google Scholar]
- Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51-75.
- [Google Scholar]
- Regulation and modulation of oocyte maturation in the bovine. Theriogenology. 1997;47:13-22.
- [Google Scholar]
- The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum Reprod. 2011;26:1270-83.
- [Google Scholar]
- Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20:1534-40.
- [Google Scholar]
- In vitro maturation versus IVF with GnRH antagonist for women with polycystic ovary syndrome: Treatment outcome and rates of ovarian hyperstimulation syndrome. Reprod Biomed Online. 2014;29:545-51.
- [Google Scholar]
- Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod Biomed Online. 2014;28:284-99.
- [Google Scholar]
- In vitro follicle growth: Achievements in mammalian species. Reprod Domest Anim. 2001;36:3-9.
- [Google Scholar]
- Primate and bovine immature oocytes and follicles as sources of fertilizable oocytes. Hum Reprod Update. 2000;6:457-74.
- [Google Scholar]
- Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032-6.
- [Google Scholar]
- Culture of oocytes and risk of imprinting defects. Hum Reprod Update. 2013;19:52-66.
- [Google Scholar]
- Assisted reproduction treatment and epigenetic inheritance. Hum Reprod Update. 2012;18:171-97.
- [Google Scholar]
- Chromosome abnormality rates in human embryos obtained from in-vitro maturation and IVF treatment cycles. Reprod Biomed Online. 2010;21:552-9.
- [Google Scholar]







