Translate this page into:
Ovarian interleukin profile and pregnancy outcome in women undergoing assisted reproduction: A prospective study
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Ovarian interleukins (ILs) mediate folliculogenesis, gametogenesis, fertilization, embryo development and implantation.
Aims:
Evaluation of the role of the quantitative levels of follicular fluid (FF) IL-1Beta (1β), IL-10 and IL-12B (p-40 subunit) in women underwent assisted reproduction.
Setting and Design:
Prospective observational study conducted between July 2013 and August 2015 at University Hospital’s in vitro fertilization (IVF) set up in North India.
Materials and Methods:
Women (n = 168) were worked up for IVF/intracytoplasmic sperm injection-embryo transfer (ICSI-ET) cycles. FF samples were pooled and collected from ovarian follicles of size ≥16 mm for each woman on the day of oocyte retrieval. Quantitative levels of IL-1β, IL-10 and IL-12B were estimated by enzyme-linked immunosorbent assay technique. Quantitative levels along with demography, cycle characteristics, endometrial thickness, number of retrieved oocytes, fertilization rate and embryos quality were compared between pregnant and nonpregnant groups of women. Student T-test, Mann–Whitney U-test, chi-squares test and logistic regression were applied as appropriate. Statistical significance level was calculated at P < 0.05.
Results:
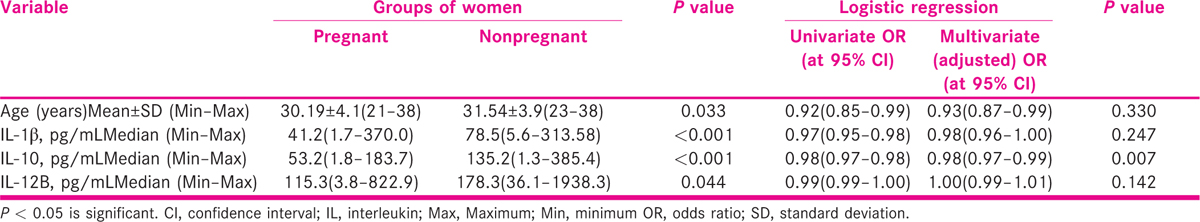
Women (n = 168) were divided into pregnant (Group A; n = 75) and nonpregnant (Group B; n = 93) groups. The median levels of IL-1β, IL-10 and IL-12B levels were found significantly lower in women in Group A as compared to Group B [41.2 pg/mL (1.7–370) vs. 78.5 (5.6–313.58); P < 0.001**, 53.2 pg/mL (1.8–183.7) vs. 135.2 pg/mL (1.3–385.4); P < 0.001** and 115.3 pg/mL (3.8–822.9) vs. 178.3 pg/mL (36.1–1938.3); P < 0.009*, respectively].
Conclusion:
Lower concentrations of IL-1β, IL-10 and IL-12B in FF were found in a protagonist with positive pregnancy outcome and can be served as a reliable predictive marker of successful IVF/ICSI-ET outcome in women underwent assisted reproduction.
Keywords
Cytokines
embryo transfer
interleukins
in vitro fertilization
ovary
pregnancy outcome
INTRODUCTION
Repeated implantation failure (RIF) is a concomitant aspect of assisted reproduction, which is surprisingly not well understood.[1,2] The contribution of immune system attained intensified recognition in the regulation of all ovarian functions including implantation.[3,4] RIF is also found associated with ovarian deregulation, complications generated during systemic events and immunomolecular mechanism in assisted reproduction.[5,6] Ovarian interleukins (ILs) are secreted by granulosa cells and other immune cells within ovary and the follicles.[7] ILs mediate intraovarian events to regulate folliculogenesis, gametogenesis, ovulation, fertilization, normal embryo development, endometrial receptivity and implantation.[8,9,10,11,12,13,14]
Present study was conducted to address the possible role of (IL)-1Beta (1β), IL-10 and IL-12B in clinical pregnancy outcome in vitro fertilization/intracytoplasmic sperm injection-embryo transfer (IVF/ICSI-ET) cycles.
MATERIALS AND METHODS
This prospective observational study was conducted between July 2013 and August 2015 at a tertiary care IVF center in north India. Informed consent forms were obtained from each women/couple participated in the study. Approval of Institutional Ethics Committee (IEC) of medical college and associated hospitals was obtained (No. F.2./IEC/MAMC/09/No.196/Dated 4th December 2009). Present study was submitted to Clinical Trial Registry, India (CTRI website URL: http://ctri.nic.in) to obtain a trial registry number as mandate. The reference number, REF/2015/06/009257, of the study has been provided; however, trial number is still awaited.
Sample size
A sample size including 165 women was calculated as per 30% IVF success rate, 95% confidence level with 5% error and 15% prevalence of infertility among general population.[15]
Enrollment of the participants for study
Infertile women were enrolled from “Fertility and assisted reproductive technology (ART) Clinic” OPD, who fulfilled the inclusion criteria.
Inclusion and exclusion criteria
Infertile women having age between 20 and 38 years with tubal factor, male factor, ovulatory dysfunction/polycystic ovary syndrome (PCOS), endocrinal disorders, moderate-to-severe endometriosis, pelvic inflammatory disease and unexplained infertility were included consecutively during their IVF/ICSI-ET cycles, whereas infertile women with uterine anomalies, uncompensated heart diseases and inadequate response of endometrial lining (endometrial thickness <7 mm) were excluded from the study.
Work-up of the women for in vitro fertilization/intracytoplasmic sperm injection-embryo transfer cycles
The women were undergone various investigation (general and specific) to rule out the cause of infertility. The women were called on day-2/3 of their natural menstrual cycle for the estimation of levels of baseline hormonal profile.
Husband’s semen samples were analyzed as per WHO-2010 criteria.[16] On the basis of investigations, women were found to have different etiologies such as tubal factor, male factor, ovulatory dysfunction (PCOS), unexplained, endocrinal disorders, endometriosis and diminished ovarian reserve (DOR).
The women were worked up, and they consecutively underwent IVF/ICSI-ET cycles according to the hospital’s protocols. The treatment cycles of the women were managed either by downregulation of pituitary with long gonadotropin-releasing hormone agonist (GnRHa) or by GnRH antagonist protocol of ovarian stimulation. Human chorionic gonadotropin (hCG, Fertigyn; Sun Pharmaceutical Ind. Ltd., Halol, Baroda, Gujarat, India) or GnRH agonist (GnRHa, Leuprolide acetate: Luperide; Sun Pharmaceutical Ind. Ltd., Halol, Baroda, Gujarat, India) was administered to trigger the final maturation of oocytes(s) to Metaphase-II stage. Oocyte retrieval (ovum pick-up) was initiated 34–36 h after the trigger.
Oocyte retrieval
Ultrasound guided oocyte retrieval was consecutively performed in all the women by using 17GA/35 cm, ovum pick-up needle (ova stiff ovum aspiration needle; K-OSN-1735-B-90; William A. Cook Australia, Pty. Ltd., Brisbane, Australia). Follicles were aspirated and follicular fluid (FF) was screened under stereo-zoom microscope for the presence of oocyte-corona cumulus complex (OCCC).
Collection and storage of the follicular fluid samples
A total of 168 clean and transparent (not mixed-up with blood) human FF samples were collected during oocyte retrieval. The FF samples from individual women were pooled, centrifuged at 300×g and collected in labeled cryovial of 1.8 mL volume capacity (Tarson Products Pvt. Ltd., 31 Shakespeare Sarani, Kolkata, India) immediately after oocyte collection.
The supernatant was collected for quantitative estimation of levels of ILs. These FF samples were stored at −20°C until assaying.
Embryo culture, embryo transfer and progesterone support
OCCCs were collected, washed with fertilization media (Vitrolife, AB, Goteborg, Sweden) and incubated in culture dishes (Nunc-4 well culture dish; Thermo Scientific, USA) at humid environment of 6%CO2, 5%O2 and 37°C temperature to acclimatize in culture conditions. Sperm insemination or ICSI was performed, 2–4 h later to oocyte collection. Fertilization analysis for the presence of two pro-nuclei (2xPN) stage was conducted 16–18 h of postinsemination or ICSI. Embryos were cultured up to 2–5 days, and accordingly embryo transfers were performed depending upon of the treatment cycle of individual woman as per standard management. Embryos were scored as per embryo scoring system, and high grades of embryos were transferred to uterine cavity.[17]
Follow-up of successful pregnancies
Progesterone (Inj. Susten/Aqsusten 50 mg, intramuscularly) in oil/water on alternate days and micronized progesterone vaginal capsules 800 mg (Cap. Susten; Sun Pharmaceutical Ind. Ltd., Halol, Baroda, Gujarat, India) were provided for next 2 weeks for luteal support. Women were called after 2 weeks of embryo transfer for urine pregnancy test and estimation of serum βhCG levels. Blood samples with serum βhCG level >50 mIU/mL were diagnosed as positive pregnancy. Samples were also repeated after 48 h for doubling of serum βhCG levels for successful implantation (pregnant group). Lower βhCG levels (<50 mIU/mL) were declared as implantation failure (nonpregnant group). Transvaginal sonography was performed after 4–5 weeks of embryo transfer for the presence of gestational sac with fetal heart and pregnancy outcome was diagnosed as viable clinical pregnancies.
Estimation of the quantitative levels of interleukins in follicular fluid samples
Quantitative estimation of the concentration of ovarian ILs in human FF samples was performed with solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) technique. The collected cryopreserved samples were thawed and processed for the quantitative estimation of levels of IL-1β, IL-10 and IL-12B concentrations. The measurement was performed with ELISA diagnostic kits [IL-1β (Gen-Probe Diaclone SAS, Besancon Cedex, France, sensitivity <6.5 pg/mL, intra-assay CV 4.5% and interassay CV 8.7%), IL-10 (Gen-Probe Diaclone SAS, Besancon Cedex, France, sensitivity <5.0 pg/mL, intra-assay CV 3.2% and interassay CV 7.3%) and IL-12B (IL-12p40 subunit; Gen-Probe Diaclone SAS, Besancon Cedex, France, sensitivity <20.0 pg/mL, intra-assay CV 3.7% and interassay CV 9.4%)] and ELISA reader (BoiTek Instruments, Germany) as per user manual’s instructions. The recommended standards with specific range of measurement to assay the levels of ovarian ILs are as follows:
IL-1β: 500, 250, 125, 62.5, 32.25, 12.6, zero (blank) and control.
IL-10: 400, 200, 100, 50, 25, 12.5, zero (blank) and control.
IL-12B (IL-12p-40 subunit): 2000, 1000, 500, 250, 125, 62.5, zero (blank) and control.
The quantitative levels of respective ILs were assigned to FF samples of individual women. The levels of each IL were categories, and only detected levels of IL’s concentrations were subsequently evaluated and compared between pregnant versus nonpregnant groups of women.
Statistical analysis
Statistical analysis for quantitative levels IL-1β, IL-10 and IL-12B and their association with pregnancy outcome was conducted. Data obtained after quantitative estimation were collected and entered in a computerized database (spreadsheet). Quantitative levels of levels IL-1β, IL-10 and IL-12B were compared for differences in mean ± standard seviation (SD) and median ± standard error of mean (SEM) values with the help of Statistical Package for the Social Sciences (SPSS 21.0; IBM Corporation, New York, USA) statistical software package.
Women were divided into two groups and compared in relation to pregnancy outcome as successful implantation (pregnant women; Group A) and implantation-failure (nonpregnant women; Group B) groups.
Both the groups were analyzed and evaluated for demographic profile, cycle characteristics, hormonal levels, endometrial thickness, number of oocytes, fertilization rate, grading of embryos and prediction of pregnancy outcome based on IL levels. Student T-test, Mann–Whitney U-test, chi-squares test and logistic regression test were applied as appropriate and statistical significant level was calculated at P < 0.05.
RESULTS
All the women (n = 168) were divided into pregnant (Group A) and nonpregnant (Group B) groups. Among them, a total of 75 (44.64%) women were found pregnant, whereas 93 (55.36%) women were found unable to achieve their pregnancy. Comparison of demographic distribution and cycle characteristics between pregnant and nonpregnant groups of women is shown in Table 1.

The pregnant women were found younger (30.19 ± 4.1 ranging 21–38 years vs. 31.54 ± 3.9 ranging 23–38; P = 0.037*) in comparison to nonpregnant group of women. The mean age difference was found statistically significant. Mean body mass index was 24.75 ± 3.3 and 25.08 ± 4.1; P = 0.571 in between pregnant and nonpregnant groups of women, respectively, and was not significant.
The mean duration of infertility in pregnant group was 6.84 ± 4.1 (range 1–20 years), whereas it was 7.91 ± 3.9 (range 1–16 years) in nonpregnant group of women. The duration of infertility was marginally lower in pregnant group as compared to nonpregnant group and was found nonsignificant (P = 0.088).
The pregnant and nonpregnant groups of women were analyzed for the distribution of type of infertility. Primary infertility was present in 70.7% (53), and secondary infertility was present in 29.3% (22) in pregnant group of women, whereas in nonpregnant group, 59.1% (55) women had primary infertility, and 40.9% (38) women had secondary infertility (P = 0.121). The distribution of different etiology among the women in pregnant and nonpregnant groups was also studied and shown in Table 2.
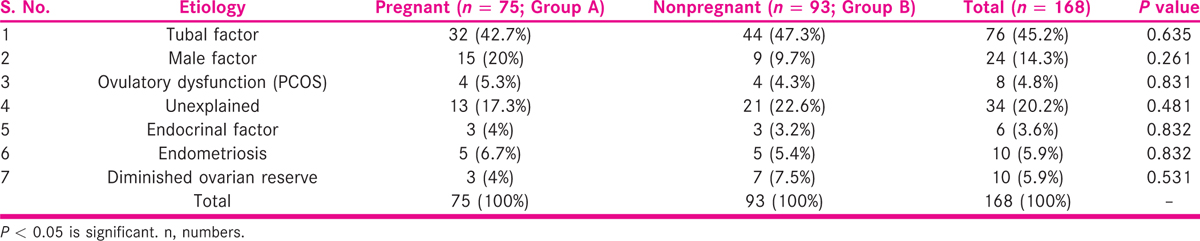
In pregnant group, 15 (20%) women were found to have male factor infertility, 32 (42.7%) women had tubal factor, 4 (5.3%) women were diagnosed with ovulatory dysfunction (PCOS), 13 (17.3%) women had unexplained infertility, 3 (4%) women were with endocrinal disorders, 5 (7.31%) women had endometriosis and 3 (6.09%) women were found to have DOR.
The distributions of various etiology factors among 93 nonpregnant women (Group B) were also analyzed. In Group B, 9 (9.7%) women were found to have male factor infertility, 44 (47.3%) women had tubal factor infertility, 4 (4.3%) women were ovulatory dysfunction, 21 (22.6%) women were with unexplained infertility, 3 (3.2%) women had endocrinal disorders, 5 (5.4%) women were with endometriosis and 7 (7.5%) women had DOR. It was observed that during the study period, women with tubal factor contributed to major proportion of patients followed by unexplained infertility enrolled for ART cycles as shown in Table 2.
No significant difference was observed when comparison of etiology was made between pregnant and nonpregnant groups.
Various characteristics of cycles, based on ovarian stimulation protocol, were compared between pregnant and nonpregnant groups.
The characteristics of the agonist and antagonist cycles were compared in terms of endometrial thickness on day of trigger, number of retrieved oocytes, number of fertilized oocytes, number of embryo transferred to uterine cavity and number embryos cryopreserved (vitrified). The results are summarized in Table 3.
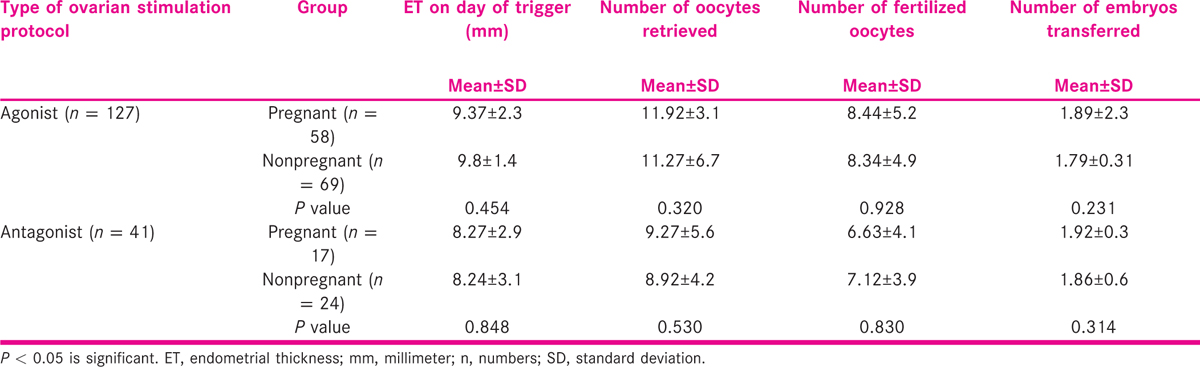
On comparison, no significant differences were observed in endometrial thickness, number of oocytes retrieved, number of transferred embryos and number of embryos vitrified, between the pregnant and nonpregnant women underwent agonist and antagonist of ovarian stimulation.
The comparison between the pregnant and nonpregnant women groups of women was made on the basis of detected levels of the ovarian ILs.
Distribution of the women was made on the basis of detected and undetected levels of the ovarian ILs (IL-1β, IL-10 and IL-12B) in pregnant and nonpregnant groups of women are shown in Table 4.
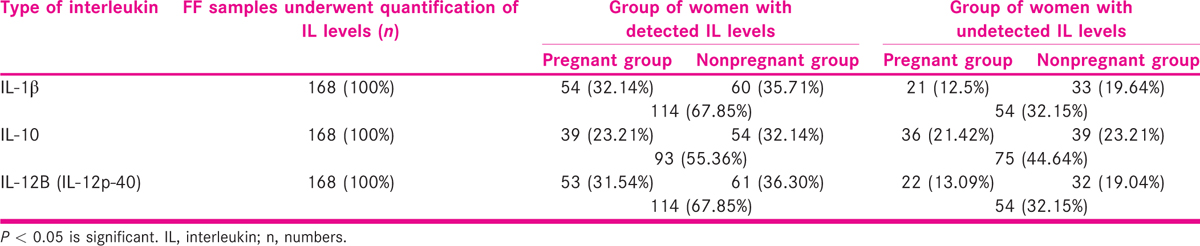
The levels were not detected in all the FF samples. It was found that IL-1β, IL-6 and IL-12B were detected in 67.85% (114) of the FF samples, whereas IL-10 was detected only in 55.36% (93) samples of the women. The undetected levels of IL-1β, IL-6, and IL-12B were observed in 32.15% (54) women, whereas IL-10 was not detected in 44.64% (75) women [Table 4].
Levels of ovarian ILs were compared between pregnant versus nonpregnant groups of women: On comparison the median values of detected levels of IL-1β, IL-10 and IL-12B were found lower in pregnant group and were highly significant statistically as compared to nonpregnant group of women as shown in Table 5.

The median levels of IL-1β, IL-10, and IL-12B were lower [IL-1β (41.2; 1.7–370 vs. 78.5; 5.6–313.58 pg/mL; P < 0.001), IL-10 (53.2; 1.8–183.7 vs. 135.2; 1.3–385.4 pg/mL; P < 0.001) and IL-12B (115.3; 3.8–822.9 vs. 178.3; 36.1–1938.3 pg/mL; P = 0.009)], respectively, in pregnant group of women as compared to nonpregnant group of women [Table 5].
Role of significant factors in prediction of pregnancy outcome
The significant levels of IL-β, IL-6, IL-10 and IL-12 were analyzed through receiver operating characteristics (ROC) curve. The ROC curve was plotted to depict area under the curve (AUC), and cut points are shown in Figures 1-3.
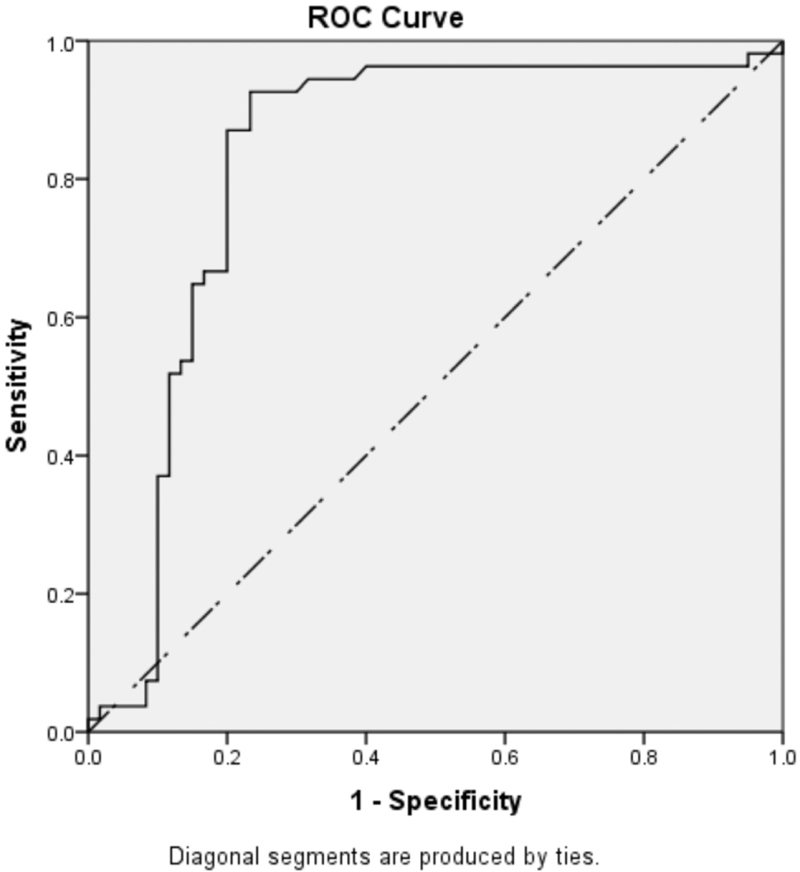
- ROC curve of the levels of IL-1β in pregnant group of women [IL-1β − cut point: <46.2; area under curve: 0.855 (85.5%)]
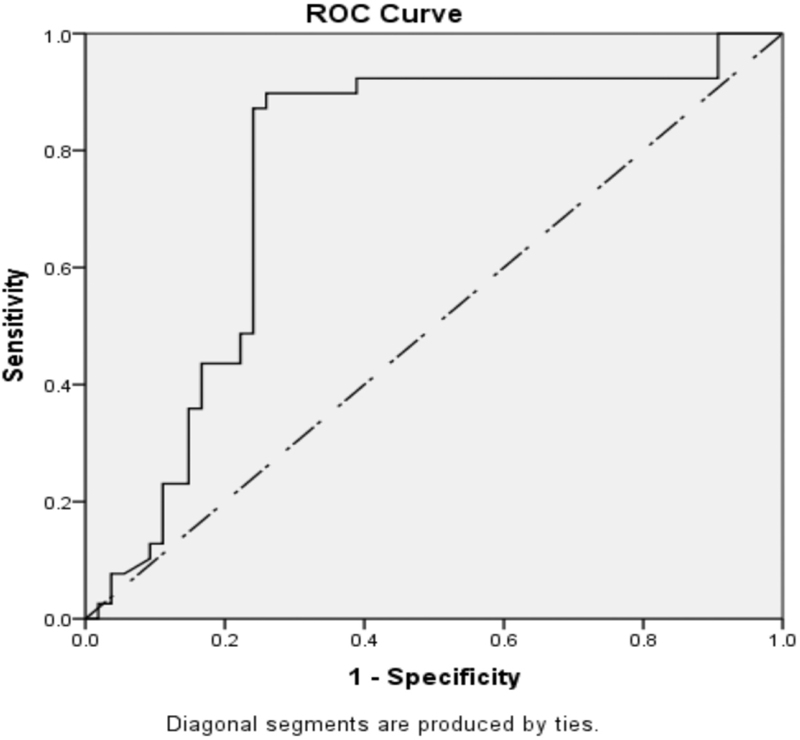
- ROC curve of quantitative levels of IL-10 in pregnant group of women [IL-10 – cut point: <62.3; area under curve: 0.760 (76.0%)]
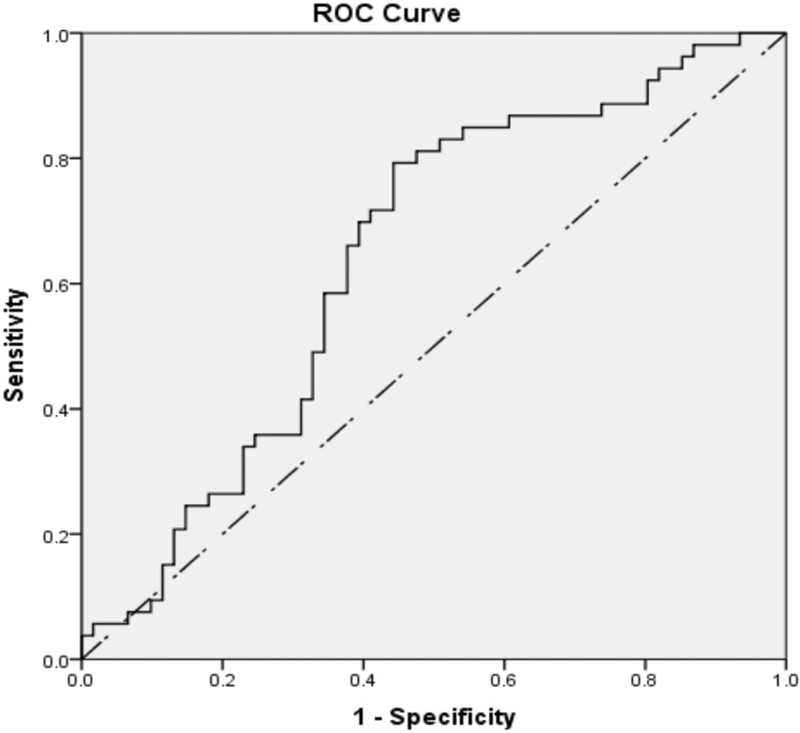
- ROC curve of quantitative levels of IL-12B (IL-12p-40 subunit) in pregnant group of women [IL-12B − cut point: <125; area under curve: 0.642 (64.2%)]
The cut-off values of IL-1β IL-10, and IL-12B with sensitivity, specificity, and AUC were calculated and summarized in Table 6.

The calculated cut-point level of IL-1β was <46.2 pg/mL with 80% sensitivity, 81.5% specificity and 82.5% AUC. Similarly, IL-10 cut-point level was <62.3 pg/mL (75% sensitivity, 76.9% specificity and AUC 76.%) and IL-12B cut-point level was <125 pg/mL (62.3% sensitivity, 62.3% specificity and AUC 64.2%). The ROC curve was plotted to predict the likelihood of successful pregnancy in relation to cut-off values of IL-1β, IL-10 and IL-12B. Positive pregnancy outcome was predicted at the respective cut-point levels of IL-1β, IL-10 and IL-12B as shown in Table 6.
Logistic regression test was applied to predict the likelihood of the pregnancy in relation to the variables, and those were found significant upon comparison between pregnant and nonpregnant groups of women.
The likelihood of the pregnancy with odds ratio (OR) at 95% confidence interval (CI) was analyzed by using enter method. Univariate logistic regression of age (OR 0.92; CI 0.85–0.99; P = 0.033), IL-1β (OR 0.97; CI 0.95–0.98; P < 0.001) and IL-12B (OR 0.99; CI 0.99–1.00; P = 0.044) was found significantly associated with successful implantation.
Multivariate logistic regression was used and adjusted for age, IL-1β, IL-10 and IL-12B. The lower levels of IL-10 (OR 0.98; CI 0.97–0.99; P = 0.007) were found associated with successful pregnancy outcome with OR at 95% CI. It was observed that per unit increase to 88 pg/mL in IL-10 levels will lead to reduction in successful implantation by 3.9% which was found as independently associated significant factor. It was observed that levels of IL-10 alone has the potential to predict the likelihood of pregnancy to 85.5% at cut-off value of <62.3 pg/mL with 75% sensitivity and 76.9% specificity as depicted in Table 7. On the basis of regression analysis, IL-10 level was found independently associated predictive marker of successful pregnancy in IVF/ICSI-ET cycles.
DISCUSSION
The ovary is a site of cytokine production, action and export into the circulation, influencing a wide variety of functions, involved in follicular development, ovulation, embryo development, luteal physiology and implantation.[18] FF is a superfluous, abundant and easily available biological material in assisted reproduction cycles, and it is considered as an optimal source to predict the association of pleiotropic noninvasive markers. As the FF provides the microenvironment for oocyte development, investigators are trying to identify predictive biomarkers in the FF that are associated with fertility-related phenotypes, including follicle development, oocyte competence, oocyte fertilization, embryo development, embryo quality, successful implantation and pregnancy outcomes.[14] Embryo quality is a key factor for successful pregnancy, and morphological characteristics and cleavage rates are currently the main clinical criteria for selecting and transferring embryos. It has been estimated that only about 5–66% and 3–55.9% of IVF cycles result in successful pregnancy and live birth rate, respectively.[19] In addition, a relationship between the metabolic profile of the FF and oocyte developmental potential and implantation outcome has been uncovered. To improve IVF outcome, one fundamental problem that needs to be solved is to accurately predict oocyte and embryo developmental potential. Because the FF affects oocyte development, its composition has been investigated as a possible predictor of oocyte and embryo quality.[20]
Previous studies have shown the relationships among growth factors,[12,21,22] proteins,[24] reactive oxygen species,[25,26] and metabolites,[27,28] in the FF and oocyte quality, fertilization rate, embryonic developmental potential and pregnancy outcome.[29] The contribution of the immune system to ovarian function is now well accepted alongside the role of gonadotropins and other intragonadal mediators.[18,30,31] There is also a possible role of the urinary and recombinant gonadotropins on different ILs levels as practiced for ovarian stimulation during assisted reproduction. These gonadotropins induce local and systemic production of ovarian ILs such as IL-1β, and IL-1α. Some authors suggested that women revealed significantly higher levels of FF IL-1β in the implantation cycles in comparison to implantation failure cycles. However, the levels of IL-1α in the two groups were found to be nonsignificant statistically.[32] Other studies showed that implantation failure was also found to be associated with deregulation of IL-12, IL-15 and IL-18.[12]
It has been found that IL-1β is an ovulation-associated cytokine,[33] and IL-8 appears to be an essential part of folliculogenesis, although its concentration is not associated with oocyte quality, fertilization or implantation rate. It was observed that women with PCOS had significantly lower level of FF IL-12 in comparison to normal ovulating women[34] and also found associated with a negative IVF outcome.[35,36] Studies showed that the quantitative levels as well as mRNA expression levels of the pleiotropic cytokines/ILs, and regulatory immune-components have their role in ovarian functions and various events during assisted reproduction.[7,13,37]A number of such reports with contradictory finding encouraged us to attempt a similar study in Indian population to find out possible participation and effect of ovarian ILs on IVF/ICSI-ET outcome. Objective of the present study was to determine the quantitative levels of ovarian ILs (IL-1β, IL-10 and IL-12B) in the stimulated preovulatory and periovulatory ovarian follicles as noninvasive and useful approach for the assessment and the prediction of the IVF-ET outcome without compromising the oocyte competence. However, the observation of present study suggested quantitative levels of IL-1β, IL-10 and IL-12B were inversely proportional to successful pregnancy outcome.
The possible reasons for such incongruous observations may be the involvement of endocrinal milieu, different etiologies, number of subjects (sample size) analyzed, response of women to gonadotropins therapy, inter and/or intra-assay approach and techniques applied for the estimation of the levels of IL-1β, IL-10 and IL-12B as discussed over.[2,37,38] Moreover, the definitive identification of key developmental proteins will provide an insight into the cellular and biochemical processes occurring during oocyte maturation, which will be crucial to determine its developmental competence including the unique molecular events that occur at the time of embryonic development and implantation.[5,12,27] A real window of application of “Omes and Omics” approach at different levels during assisted reproduction cycles for investigation, diagnostics and therapeutics and to improve IVF success rate is still unexplored.[38,39]
CONCLUSION
Age was found as paramount and significant factor associated with a positive pregnancy outcome as women who achieved their successful pregnancies were younger.
The median levels of IL-1β, IL-10 and IL-12B were significantly lower in pregnant women as compared to nonpregnant group of women. The quantitative levels of IL-1β, IL-10 and IL-12B were found associated with successful IVF/ICSI-ET outcome and also have their role as reliable predictive marker. In addition to these findings, the present study suggests that IL-10 quantitative levels are having an independent association with the successful pregnancy outcome in women undergoing assisted reproduction cycles. However, the role of ILs in the local processes of ovary is still poorly understood. These findings lead and encourage other investigators for conducting similar prospective multicentric comprehensively designed research studies on large population size for the validity of results and observations of present study and further evaluation of the molecular aspects of ovarian ILs in successful implantation and or implantation failure.
Financial support and sponsorship
The financial support in the form of doctoral fellowship (RGNF) was provided by University Grants Commission (UGC), New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors deeply acknowledge the patients for providing their consent to conduct the study. Assistance from the staff of IVF and Reproductive Biology Centre is also extremely acknowledged.
REFERENCES
- Clinical value of endometrial pinopodes detection in artificial donation cycles. Reprod Biomed Online. 2004;9:86-90.
- [Google Scholar]
- Differential expression of follicular fluid cytokines: Relationship to subsequent pregnancy in IVF cycles. Reprod Biomed Online. 2007;15:321-5.
- [Google Scholar]
- Comparative multiplex analysis of cytokines, chemokines and growth factors in follicular fluid of normoresponder women undergoing ovum donation with gonadotropin-releasing hormone agonist versus gonadotropin-releasing hormone antagonist protocols. J Hum Reprod Sci. 2013;6:205-12.
- [Google Scholar]
- Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2007;11:613-30.
- [Google Scholar]
- Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731-46.
- [Google Scholar]
- Analysis of intra-uterine cytokine concentration and matrix-metalloproteinase activity in women with recurrent failed embryo transfer. Hum Reprod. 2003;18:608-15.
- [Google Scholar]
- Interleukin-1beta and interleukin-1alpha may affect the implantation rate of patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 1998;70:553-9.
- [Google Scholar]
- Role of transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:14-23.
- [Google Scholar]
- The potential role of interleukin-1 in the ovulatory process: An evolving hypothesis. Mol Cell Endocrinol. 1998;140:77-81.
- [Google Scholar]
- Relationship between ovarian stimulation regimen and cytokine concentration in follicular fluid and their effect on fertilization and pregnancy outcome of patients undergoing ICSI program. Am J Reprod Immunol. 2000;43:12-20.
- [Google Scholar]
- Immunoglobulins and cytokines level in follicular fluid in relation to etiology of infertility and their relevance to IVF outcome. Am J Reprod Immunol. 2002;47:82-90.
- [Google Scholar]
- Role of the endometrial tripod interleukin-18, −15, and −12 in inadequate uterine receptivity in patients with a history of repeated in vitro fertilization-embryo transfer failure. Fertil Steril. 2005;83:598-605.
- [Google Scholar]
- The human ovarian follicular fluid level of interleukin-8 is associated with follicular size and patient age. Fertil Steril. 2010;93:537-43.
- [Google Scholar]
- Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40-51.
- [Google Scholar]
- Adequacy of Sample Size in Health Studies. Chichester: John Wiley & Sons Ltd; 1990.
- WHO Laboratory Manual for the Examination and Processing of Human Semen (5th). Geneva: WHO Press; 2010.
- Involvement of leukocytes and cytokines in the ovulatory process and corpus luteum function. Hum Reprod. 1993;8:1762-75.
- [Google Scholar]
- 2013. 2013 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Available from: http://www.cdc.gov/ART/PDF/ART2013_National_Summary_report.pdf.
- International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted reproductive technology2006. Hum Reprod. 2014;29:1536-51.
- [Google Scholar]
- Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil Steril. 2006;86:1392-401.
- [Google Scholar]
- Correlations between anti-mullerian hormone, inhibin B, and activin A in follicular fluid in IVF/ICSI patients for assessing the maturation and developmental potential of oocytes. Eur J Med Res. 2007;12:604-8.
- [Google Scholar]
- Ovarian follicular concentration of IL-12, IL-15, IL-18 and p40 subunit of IL-12 and IL-23. Hum Reprod. 2006;21:2650-5.
- [Google Scholar]
- Soluble CD44 in human ovarian follicular fluid. J Assist Reprod Genet. 2001;18:21-5.
- [Google Scholar]
- Reactive oxygen species level in follicular fluid − Embryo quality marker in IVF? Hum Reprod. 2006;21:2403-7.
- [Google Scholar]
- Mitochondria in human oogenesis and preimplantation embryogenesis: Engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269-80.
- [Google Scholar]
- Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction. 2013;146:389-95.
- [Google Scholar]
- Endometrial secretion analysis identifies a cytokine profile of pregnancy in IVF. Hum Reprod. 2009;24:1427-35.
- [Google Scholar]
- Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021.
- [Google Scholar]
- The potential relevance of cytokines to ovarian physiology: The emerging role of resident ovarian cells of white blood cell series. Endocr Rev. 1990;11:454-64.
- [Google Scholar]
- Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101:1177-82.
- [Google Scholar]
- Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries: Is ovulation a suppressed inflammatory reaction? Hum Reprod. 1999;14:162-6.
- [Google Scholar]
- Correlation between concentration of interleukin-12 and interleukin-13 and lymphocyte subsets in the follicular fluid of women with and without polycystic ovary syndrome. Fertil Steril. 2003;79:1365-72.
- [Google Scholar]
- Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod. 2012;27:1025-33.
- [Google Scholar]
- Follicular fluid concentration of interleukin-12 and interleukin-8 in IVF cycle. Fertil Steril. 2000;74:953-8.
- [Google Scholar]
- Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007;22:527-35.
- [Google Scholar]
- Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004;22:245-52.
- [Google Scholar]
- From global proteome profiling to single targeted molecules of follicular fluid and oocyte: Contribution to embryo development and IVF outcome. Expert Rev Proteomics. 2015;12:407-23.
- [Google Scholar]







