Translate this page into:
Comparison of physiological-ICSI (PICSI) with ICSI in cases of moderate to severe oligoasthenoteratozoospermia (OAT) − a randomized control study
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To compare the efficiency of physiological intracytoplasmic sperm injection (PICSI) over intracytoplasmic sperm injection (ICSI) in Oligoasthenoteratozoospermic patients.
Design:
Randomized control study.
Setting:
KJIVF and laparoscopy center Delhi.
Methods:
From September 2019 to May 2020, 45 patients of male factor felled under our criteria of OAT were divided into two groups of PICSI and ICSI. Sperm selection was performed under high magnification in both the groups and results were compared.
Outcome measure:
Primary outcome: Fertilization rate, cleavage rate, blastulation, and utility rate; Secondary outcome: Clinical pregnancy rates and miscarriage rates.
Results:
20 PICSI and 25 ICSI treatments were performed and observed that the rate of blastulation and embryo utility shows significant difference in PICSI group (P = 0.013). The pregnancy percentage was also better in PICSI but implantation, fertilization, cleavage; clinical pregnancy rates were clinically comparable in both the groups but not statistically significant.
Conclusion:
The outcomes of our study are independent of male factor and it only depends on intervention whether it was PICSI or ICSI. The blastulation rate and embryo utility shows statistically significant difference between PICSI and ICSI groups. Hence, we are slowly progressing toward the superiority of PICSI over ICSI but enough evidences are still not available.
Keywords
Blastulation
fertilization
intracytoplasmic sperm injection
physiological intracytoplasmic sperm injection and morphology
INTRODUCTION
The 10% to 15% of total population is affected by infertility and the male factor is solely responsible in 40%.[1,3]. Intracytoplasmic sperm injection (ICSI) was introduced to increase the pregnancy in severe male infertility.[2,5,13] ICSI children born is around 10 per 1000 with live birth rates of 24% and remained unchanged in decades.[14,16,22,23] Toxicity of PVP affects rate of fertilization and sperm head decondensation.[4,26,27] The oocyte is surrounded by HA; a high-molecular weight glycosaminoglycan and basis is a specific receptor that binds to HA is present on the head of mature sperms.[5,17,20] There is no known adverse impact on zygote development.[20] This study is conducted to determine the efficacy of physiological intracytoplasmic sperm injection (PICSI) over ICSI in OAT.
MATERIALS AND METHODS
The randomization of 25 ICSI cycle with 20 PICSI cycle was conducted with the help of ‘Random number generation’ App, assigning 25 & 20 subjects to each treatment group.
Design of study: Randomized control study
Place of study: KJIVF and Laparoscopy centre Delhi
Period of proposed study: 9 months from September 2019 to May 2020.
Sample size: All the subjects with male factor infertility who took consultation with us were the possible participants of our study. A total of 45 subjects during the 9 months of study fulfilled our criteria. All the 45 subjects consented and participated in the study. The participants were randomly divided into experimental [A group] and controlled group [B group].
Inclusion criteria: Patients with male factor infertility (moderate to severe OAT) were selected.
Count: The criteria for OAT sperm concentration 1×106/mL to 10×106/mL, motility ≥5% and <40%, and strict morphology < 4%.
Exclusion criteria: Patient with other compounding factor like decreases ovarian reserve, metabolic and endocrinal problem and testicular sperm.
Outcomes of study ⇒ analyzed: Primary outcomes: Fertilization rate, cleavage rate, blastulation, and utility rate
Secondary outcomes: clinical pregnancy rates and miscarriage rates.
The embryologist performing the ICSI must be experienced, to avoid any variability between different operators. All patients were aware of the procedure and have signed the written consent.
This study was approved by the ethical committee of IFS.
Ovarian stimulation, oocyte retrieval and selection
GNRH analogs in combination with a graded menotropin controlled ovarian stimulation in women were carried out. When the size of two or more follicles reached 18 mm, a dose of 10,000 IU of human chorionic gonadotropin (HCG) was given and transvaginal ultrasound-guided oocyte retrieval was preformed 36 hours after HCG trigger.
The oocytes were incubated for 1 to 2 hours. At 37°C in 6% CO2 and 5% O2, and 89% N2 in Heracell incubator before complete removal of cumulus mass and corona cells in 80 IU hyaluronidase (Sage) by gentle mechanical aspiration with denuding pipettes.
Sperm preparation
The semen collection was done in sperm wash medium to avoid damage caused by the seminal fluid factors in cases of poor semen parameters to get good viable sperms,[7] before the process of denudation in a well-labelled sterile container.
After proper liquification of sample the spermatozoa were treated by density gradient (80% and 40% Sage density gradient and HEPES flushing medium) method according to World Health Organization (WHO) guidelines.[28]
Before each procedure an embryologist checks the randomization with the help of app “Random number Generation” to assign the patient for PICSI or ICSI group.
Conventional intracytoplasmic sperm injection
After denudation the eggs were checked for M1, polar body and M2 stage and were cultured in HEPES medium for 1 to 2 hours. The ICSI dish was prepared as shown in Figure 1. The ICSI procedure was carried out on heated stage of an inverted Olympus microscope equipped with Narishige micromanipulator (Narishige, Tokyo, Japan) under 200x to 400x magnifications. The sperms with normal morphology were selected and injected into the mature oocytes.
- Preparation of intracytoplasmic sperm injection Dish: a 5 µl drop of polyvinylpyrrolidone for priming the injrction pipette. 5 µl HEPES medium drops for keeping the oocytes during ICSI. The number of HEPES drops depends on the number of oocytes to be injected. Everything is covered under oil
PICSI procedure
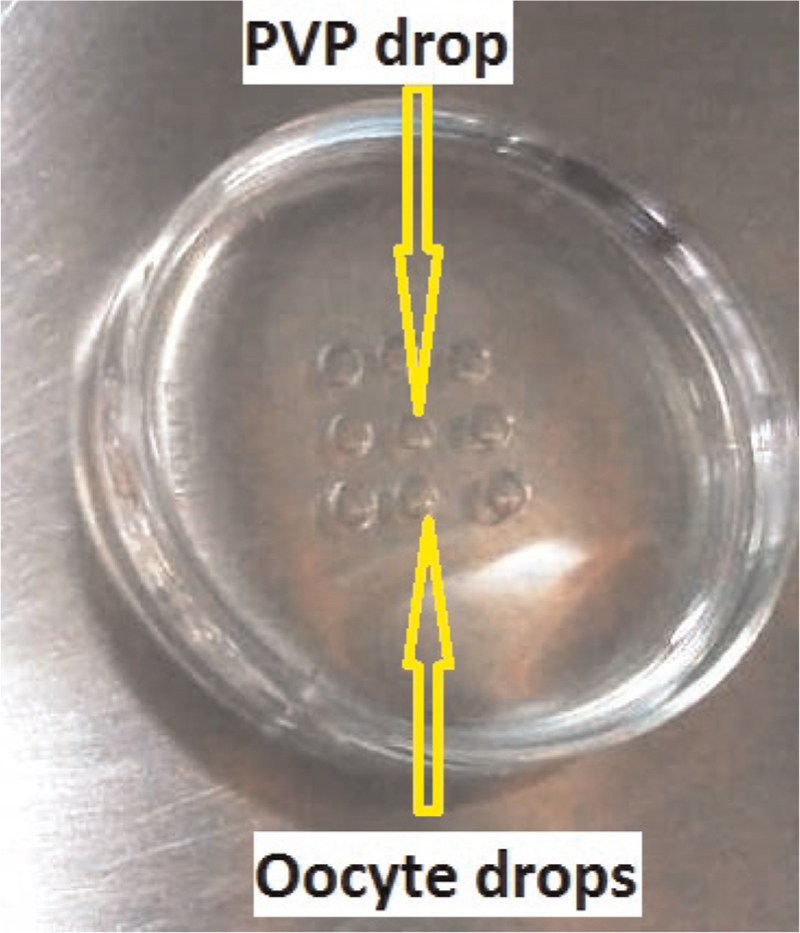
For PICSI conventional plastic culture dishes prepared with three microdots of powdered HA. By adding 5 mL droplet (in a long-tail shape) of fresh culture medium (flushing medium HEPES) to each of the three microdots the powered HA was rehydrated. Near each 5 mL culture medium droplet (tail-shaped) a 2 mL droplet with suspension of treated spermatozoa was placed and connected to the droplet using tip of the pipette [Figure 2]. The PICSI dish was incubated at 37°C under oil; within 5 minutes the mature sperms binds by their head to the surface of HA microdots and were spinning around their head. Spermatozoa spinning faster were preferred and with ICSI injecting pipette HA-bound sperm was picked up and injected one by one into an oocyte. Both PICSI and ICSI were performed at x400 magnification; the spermatozoa were selected for their morphology according to WHO2010 guidelines.

- Preparation of physiological intracytoplasmic sperm injection dish: one 5 µl drop of polyvinylpyrrolidone for priming the injection pipette in the centre of oocytes micro drop. Three tail shaped 5 µl drops to rehydrate dry hyaluronan microdots; 5 µl HEPES drops for keeping the oocytes during ICSI. The number of HEPES drops depends on the number of oocytes to be injected. Everything is covered under oil
Embryo culture and transfer, main outcome measures
The injected oocytes were cultured in cleavage medium (Medi cult − origio) at 37°C in 6% CO2, 5% O2, and 89% N2 in planer benchtop incubators (origio). The inverted microscope with grading 1 to 5, that is (best to worst) based on the criteria mentioned in “Gardner” was used for checking fertilization and cleavage of embryos. After 3 or 5 days since oocyte retrieval ET was carried out if good quality embryos were less than or equal to three. On Day 3 not more than three embryos per patient were transferred and on Day 5 two embryos were transferred and the remaining good quality embryos were vitrified.
β-HCG test was done after 17 days in Day 3 transfer and after 15 days in Day 5 transfer. The clinical pregnancy was decided by ultrasound examination a week after positive β-HCG. The primary and secondary outcomes were analyzed.
Statistical analysis
For statistical analysis, data after collection was entered in Microsoft excel and analysis was done in statistical software SPSS v 20.0 (IBM). Data thus analyzed was presented in the form of tables and diagrams. Categorical data was presented as frequency/percentage and compared using Chi square and Fisher exact tests while as continuous data was presented as mean ± SD and compared using independent T test. Risk was calculated as odds ratio with 95% confidence interval. For any observed difference P value of < 0.05 was considered as statistically significant.
RESULTS AND OBSERVATIONS
The result of study is analyzed between 20 PICSI and 25 ICSI treatments.
In general characteristics [Table 1 and Figure 3] of patients the mean age and standard deviation are almost similar between both the groups (30.10 ± 4.983) and is not statistically significant (P = 0.584). The experimental group has slightly more prevalence of addiction (smoking, alcohol, and tobacco) 20% than control group 12.0% but overall the P value is not significant (P=0.682). The moderate male factor prevalence is more in ICSI group 60.0% (45.0% in PICSI group) and severe male factor is lightly more in PICSI 55.0% (40.0% in ICSI), although P value is not significant (P = 0.337). Stimulation wise two groups are similar (P = 0.105).


- Comparison between general characteristics of PICSI and ICSI groups
The P value is not significant in any of the studied outcomes [Table 2]: no. of follicles (P = 0.056), sperm count (P = 0.185), motility (P = 0.511), no. of egg (P = 0.436).

Table 3 shows no statistical significance difference in any of the outcomes: no. of eggs injected (P = 0.693), fertilization (P = 0.745), cleavage (P = 0.602), and embryo transfer (P = 0.625). The mean blastocyst (PICSI = 3.50 and ICSI = 1.87) and mean embryo freezing rate (PICSI = 4.89 and ICSI = 2.35) is better in PICSI group and shows statistically significant difference (P = 0.013) between the two group.

The clinical pregnancy [Table 4; Figure 4] is also not statistically significant (P = 1) between both the groups but the percentage of pregnancy is better in PICSI group 40.0% with only 36.0% in ICSI group.


- Comparison of pregnancy outcomes between both the groups
Outcomes based on moderate and severe male factor in ICSI and PICSI group [Tables 5-7 Figures 5 and 6] shows no significant difference between both the groups. The pregnancy outcome based on male factor in ICSI group [Table 6] is almost similar but it is slightly more in severe male factor (40.0%) than moderate male factor (33.3%) although it is statistically not significant. Similarly, the percentage of moderate male factor pregnancy outcome [Table 8] is more (44.4%) than severe male factor (36.4%) in PICSI group, but this is also not significant (P = 1.000).





- Comparison of pregnancy with respect to severe and moderate male factors in control group

- Comparison of pregnancy with respect to severe and moderate male factors
DISCUSSION
In ICSI, the chances of aneuploidy and chromosome aberration is high as sperms are chosen based on their morphology and this increases the chances of fertilization of oocyte with damaged DNA which may lead to pregnancy loss.[11,19] The common risks associated with ICSI are damage to embryos, multiple pregnancy, and complications during pregnancy and child birth; whereas defects in ICSI occur in far less than 1% of ICSI babies.[2,24]
Previously, Strehler et al. (1998) in a study of effects of PVP on spermatozoa found that the acrosome, plasma membrane and mitochondria are the most affected components of sperm. It is also observed that after the process egg penetration PVP hinder nuclear sperm decondensation. In this study the nucleus is most damaged component both in context to shape and structure of chromatin.[25] Many studies have shown correlation between higher DNA fragmentation rates in teratozoospermic men than normozoospermic men. The head defects like tapered head, increased head length with slightly change in width, decreased acrosome coverage, all result in chromosomal aneuploidy and poor chromatin packaging.[6,24] The shape of sperm does not define absence or presence of chromosomal abnormalities; thus it is invalid criteria for selection of mature sperm devoid of chromosomal degradation.[12] The HA-bound spermatozoa shows reduced chromosomal aneuploidies and DNA fragmentation and results in good embryo quality.[26] The use of HA do not cause any toxicity to the sperm and to the oocyte, as it is a part of female reproductive tract and cumulus complex. HA is carried into oocyte during in-vivo-conception by spermatozoa therefore HA-sperm selection is safer than ICSI selection.[9,21]
The spermatozoal hyaluronidase is primarily located at the regions of plasma and inner acrosomal membranes; however PH-20: a bifunctional protein present on the sperm plasma membrane has a hyaluronidase activity and role in secondary sperm-zona binding is produced during the exocytosis of acrosome.[8,15] The hyaluronidase activity is depicted by the N-terminal domain of PH-20, which enables the penetration of cumulus cell surrounding the oocyte.[18,29]
Some studies have shown HA-sperm selection improves blastocyst rate by PICSI fertilization,[4] pregnancy rate,[10,30] decreased fragmentation rate in embryos,[30] miscarriage rate[30] when compared with conventional ICSI. Some authors also found no statistical differences in fertilization, pregnancy, implantation rates, and in blastocyst rates.[5,16]
In our study the general characteristics [Table 1 and Figure 3] of patients like age, addiction, diagnosis of moderate and severe male factor and stimulation, that is, agonist and antagonist between two groups PICSI and ICSI is compared and analyzed and found no age difference between two groups, that is, the mean age and standard deviation are almost similar in both the groups (30.10 ± 4.983) and is not statistically significant (P = 0.584). So there is no effect of age in the outcomes of result between two groups and age is not a concern may be due to small sample size. The PICSI group has slightly more prevalence of addiction (20%) than ICSI group (12.0%) but the P value is not significant (P = 0.682); so the two groups are also similar in addiction wise. The moderate male factor prevalence is more in ICSI group 60.0% (45.0% in PICSI group) but severe male factor is lightly more in PICSI 55.0% (40.0% in ICSI) although P value is not significant (P = 0.337) and stimulation wise also two groups are similar (P = 0.105). Thus, overall there is no effect of age, addiction, diagnosis, and stimulation protocol on the outcome of our study.
In Table 2, the number of variables which are almost similar in both the groups and the P value is not significant in any of the outcomes, that is, No. of follicle (P = 0.056), sperm count (million/mL) (P = 0.185), motility (%;P = 0.511), no. of egg (P = 0.436) between the two groups. Thus, two groups are comparable to each other and this is in favor of our study.
When primary and secondary outcomes are compared [Table 3] found that there is no statistical significance difference in any of the outcomes: no. of eggs injected (P = 0.693), fertilization (P = 0.745), cleavage (P = 0.602), embryo transferred (P = 0.625); but the mean blastocyst (PICSI = 3.50 and ICSI = 1.87) and mean embryo freezing rate (PICSI = 4.89 and ICSI = 2.35) are better in PICSI group with statistically significant difference (P = 0.013).
The result of clinical pregnancy [Table 4 and Figure 4] also shows no statistically significant difference (P = 1.000) between two groups but the pregnancy percentage is better in PICSI group 40.0% with only 36.0% in ICSI group. The odd ratio 1.185 shows that pregnancy rate 1.185 times more in PICSI compared to ICSI group
The outcomes based on moderate and severe male factor in ICSI and PICSI groups [Tables 5-7] shows no significant difference between two groups; this means outcomes hardly depends on whether the male factor is moderate or severe and thus the outcome in independent of male factor and is only depends on intervention whether it was PICSI or ICSI.
The pregnancy outcome based on male factor in ICSI group [Table 6 and Figure 5] is almost similar but it is slightly more in severe male factor (40.0%) than moderate male factor (33.3%); although it is statistically not significant between two groups of male factor.
Similarly, the percentage of moderate male factor pregnancy outcome [Table 8 and Figure 6] is more (44.4%) than severe male factor (36.4%) in PICSI group, but it is also not significant (P = 1) between the two groups of male factor.
CONCLUSION
The conclusion of our study is that PICSI gives good outcomes in blastulation rate and embryo utility (embryo freeze) are statistically significant in PICSI versus ICSI. Pregnancy percentage was better in PICSI group; although it was not statistically significant. The outcomes of this study are independent of male factor and only depend on intervention whether it was PICSI or ICSI.
Hence, we are slowly progressing toward the superiority of PICSI over ICSI but enough evidences are still not available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ACKNOWLEDGEMENT
I would like to express my sincerest gratitude and appreciation to my supervisor Dr. Kuldeep Jain, co-supervisor Dr. Bharti Jain and senior embryologist Mr. Shyam Shankar for exceptional guidance and constant support throughout my work. I am truly grateful for sharing their excellent knowledge and clear vision in research with me for their invaluable guidance, encouragement, patient, and support during my course.
REFERENCES
- Causes and risk factors for male factor infertility in Nigeria: a review. Afe Reprod Health. 2013;17:150.
- [Google Scholar]
- Intracytoplasmic sperm injection (ICSI) what are the risk? Urol Clin North AM. 2008;35:277-288.
- [Google Scholar]
- Epidemiology of infertility: social problems of the infertile couples. WAJM. 2003;22:190-96.
- [Google Scholar]
- An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porcine Health Manage. 2018;4:16.
- [Google Scholar]
- Physiological ICSI (PICSI) vs conventional ICSI in couples with male factor: a systematic review. JBRA Assist Reprod. 2018;22:139-147.
- [Google Scholar]
- Sperm DNA fragmentation is significantly increased in those men with morphologically abnormal. Spermatozoa JFIV Reprod Med Genet. 2014;2:131. doi: 10.4172? 2375-4508.1000131.
- [Google Scholar]
- Can intracytoplasmic morphologically selected spermatozoa injection be used as first choice of treatment for severe male factor infertility patients? J Human Reprod Sci 2018:40-44.
- [Google Scholar]
- Sperm surface protein PH-20 is bifunctional; One activity is a hyaluronidase and a second, distinct activity is required in secondary sperm-zona binding. Biol Reprod. 1996;55:80-86.
- [Google Scholar]
- Fertility testing and ICSI sperm selection by hyaluronic acid binding; clinical and genetic aspects. Reprod Biomed. 2007;14:650-63.
- [Google Scholar]
- The evaluation of sperm’s hyaluronic acid binding ability as a predictive value for the clinical success of intracytoplasmic sperm injection. Bullet Osaka Med Coll. 2019;65:13-18.
- [Google Scholar]
- Intracytoplasmic sperm injection: a novel selection method for sperm with normal frequency of chromosomal aneuploidies. Fertil Steril. 2005;84:1665-73. doi: 10.1016?J.fertnstert.2005.05.068
- [Google Scholar]
- Comparison between intracytoplasmic sperm injection and intracytoplasmic morphologically selected sperm injection in oligo-astheno-teratozoospermia patients. Clin Exp Reprod Med. 2014;41:9-14.
- [Google Scholar]
- Micromanipulation in assisted reproductive technologies reproductive. Biomed Online. 2016;32:339-347.
- [Google Scholar]
- A plasma membrane associated hyaluronidase in localized to the posterior acrosomal region of stallion sperm and in associated with spermatozoal function. Biol Reprod. 1999;61:444-51.
- [Google Scholar]
- Physiological hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HAB Select) : a parallel, two group, randomized trial. Lancet. 2019;393:416-22.
- [Google Scholar]
- Is sperm hyalironicacid binding ability predictive for clinical success of intracytoplasmic sperm injection: PICSI vs ICSI? Syst Biol Reprod Med. 2014;60:6. 348-54. doi: 10.3109/19396368.2014.948102.
- [Google Scholar]
- Why did the sperm cross the cumulus? To get to the oocyte. Functions of the sperm surface protein PH-20 and fertilin in arriving at, and fusing with, the egg. Biol Reprod. 1997;56:320-7.
- [Google Scholar]
- New Advances in Intracytoplasmic Sperm Injection (ICSI) In: Wu B, ed. Advances in Embryo Transfer. In tech; 2012. ISBN: 978-953-51-318-0
- [Google Scholar]
- Comparision of two ready to use systems designed for sperm hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI Vs Sperm slow: a prospective, randomized trial. American society for reproductive medicine. Fertil Steril. 2012;98:632-7.
- [Google Scholar]
- Efficiency of hyaluronic acid (HA) sperm selection. J Assist Reprod Genet. 2010;27:13-16.
- [Google Scholar]
- The ICSI procedure from past to future; a systematic review of the more controversial aspects. Human Repro Update. 2016;22:194-227.
- [Google Scholar]
- In vitro fertilization (IVF) and intra-cytoplasmic sperm injection (ICSI). FEr-FEB-15-ANZ-0005.2015/TAPS CH3708
- 2017. Male infertility; evidences, risk factors, causes, diagnosis and management in human. Ann Clin Lab Res. 2017;5:188.
- [Google Scholar]
- Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa. Hum Repro. 1998;13:120-2.
- [Google Scholar]
- The efficiency of conventional microscopic selection is comparable to the hyaluronic acid binding method in selecting spermatozoa for male infertility patients. Taiwan J Obstet Gynecol. 2015;54:48-53.
- [Google Scholar]
- Prevalence of low sperm count and abnormal semen parameters in male partners of women consulting at infertility clinic in Abakaliki, Nigeria after reproduction health. Afr J Reprod Health. 2008;12:67-73.
- [Google Scholar]
- WHO laboratory manual for the examination and processing of human semen. 2010;5:271. ISBN:9789241547789
- Hyaluronic acid binding sperm selection for assisted reproduction treatment (HAB select): study protocol for a multicentre randomised controlled trial. BMJ Open. 2016;6:e012609. doi: 10.1136/ bmjopen −2016-012609.
- [Google Scholar]
- PICSI VS ICSI: statistically significant improvement in clinical outcomes in 240 in vitro fertiliozation (IVF) patients. Fertil Steril. 2007;88:133. doi: 10.1016/j.fertnstert.2007.07.133
- [Google Scholar]







