Translate this page into:
A study on the impact of elevated estradiol levels in frozen-embryo transfer cycles on pregnancy rates
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims and Objectives:
To determine whether raised estradiol (E2) levels administered before progesterone supplementation in frozen-embryo transfer cycles have an impact on the rates of pregnancy.
Materials and Methods:
A retrospective study of 242 patients who had their frozen embryo replacement cycles conducted at Southend Fertility and IVF Centre between September 2014 and April 2017. In all these patients, a baseline pelvic scan was carried out and endometrial preparation was carried out as per six different protocols. After desired endometrial lining was observed, E2 levels were measured prior to progesterone supplementation, and the impact of raised E2 levels on pregnancy outcomes was evaluated in six different protocols. Pregnancy outcome was evaluated by measuring serum beta human chorionic gonadotropin levels 16 days after the embryo transfer. Statistical analysis was performed by using standard methods and receiver operating characteristic curves plotted to compare the outcomes.
Results:
Mean age of the patients in the study group was 34.35 ± 6.12 years, most of the patients were less than 40 years of age and mean parity was 0.49 ± 0.55. E2 level was measured on day 2 or 3 of cycle, and mean E2 level was 11.83 ± 5.50 pg/ml. Endometrial preparation was performed with injectable or oral hormonal preparations using six different protocols. Mean peak E2 level was 1263.65 pg/ml in 149 patients with no pregnancy and 1445.01 pg/ml in 93 patients with pregnancy in all protocols.
Conclusion:
Elevated E2 levels had no statistically significant impact on pregnancy rates in six different study protocols (oral and injectable) used for endometrial preparation.
Keywords
Estradiol levels
frozen-embryo transfer cycles
pregnancy
progesterone supplementation
INTRODUCTION
Introduction of the embryo cryopreservation technique has revolutionized the field of assisted reproductive technology (ART). Trounson and Mohr,[1] in the year 1983, proved that frozen embryos can survive and create pregnancies. Since then, many patients have utilized this technology to achieve pregnancy either when fresh transfer has failed or for subsequent pregnancies following successful in vitro fertilization (IVF). Transfer of frozen embryos has been increasingly used during the past few decades, as is an integral part of ART in the present times.[2]
Frozen-embryo transfer (FET) has several advantages and among them, the similar or even higher pregnancy and live-birth rate compared to fresh cycles is of great importance.[3] In some randomized trials and meta-analyses, higher pregnancy rate was reported after FET when compared with fresh cycles.[4,5,6] The other benefit of FET is possibility of embryo transfer in a natural, nonstimulated cycle. It is shown that an artificial cycle may adversely affect endometrial receptivity leading to implantation failure.[7,8,9,10,11] FET is also a useful method for preserving extra good quality embryos in women with good response to ovarian stimulation, increasing elective single embryo transfer and avoiding multiple gestation as well as ovarian hyperstimulation syndrome.[12] The FET is a procedure used for the transfer of excess embryos obtained during IVF/intracytoplasmic sperm injection (ICSI), and it prevents embryo wastage and increases the probability of pregnancy in a single stimulated cycle. Pregnancy rates following FET have been found to be higher than those of freshly prepared embryos, as it increases the cumulative pregnancy rates and decreases the cost; it is easy to perform and can be applied in shorter time duration.[13]
The success of a FET cycle is linked to the synchronization between endometrial maturation and embryo development, and this synchronization can be achieved in a natural modified cycle or after endometrial preparation with exogenous steroids.[14,15,16] In artificial endometrial preparation, estradiol (E2) is started in early follicular phase (day 2/day 3) to inhibit spontaneous ovulation.[17] Steroid hormones, secreted by the ovary, have multiple effects on the endometrium, resulting in proliferation and differentiation of the tissue, enhancing receptivity to embryonic implantation, and shedding in the absence of pregnancy. In the follicular phase, E2 secreted by growing follicles stimulates estrogen receptor (ER expression) and the highest levels observed in glandular epithelium during the late follicular phase.[18,19,20,21,22] The peak expression in human endometrium induced by E2 is observed at the time of ovulation.[23,24]
The E2 is essential for endometrial growth and for enabling progesterone to act on the tissues. E2 induces PR expression and promotes cellular proliferation in the tissue − directly through its cognate receptors, and indirectly by induction of growth factors that act as autocrine and/or paracrine modulators.[25]
A receptive endometrium should reach a thickness of at least 7 mm.[26] Once this thickness is achieved, above this threshold, endometrium can maintain a receptivity for embryo implantation, upto 40 to 60 days.[27,28] A shorter duration of endometrial preparation, that is, a proliferative phase less than 10 days are related to a higher abortion rates.[29] Therefore, optimal duration of endometrial preparation is a prerequisite for the optimal development of progesterone receptors and subsequent transformation into a receptive endometrium. Once the endometrium has reached an adequate thickness, there is a window of several days in which to perform the embryo thawing followed by the transfer procedure.[30]
The highest pregnancy rates in ART are obtained in fresh oocyte donation cycles and in these cycles, the endometrium is primed artificially and the embryos are therefore transferred to an environment that had not suffered the effects of the supraphysiologic hormonal levels that occur during controlled ovarian hyperstimulation (COH).[31] Although the oocytes are of the same quality, some studies of shared oocyte cycles found significantly higher pregnancy rates in recipients compared with oocyte donors, and this may be related to a superior quality of endometrial receptivity. Endometrial priming in FETs may be achieved with the use of E2 and progesterone, and the endometrial development can be controlled more precisely than in cycles of COH with gonadotropins.[32,33]
The aim of the present study was to study the relationship between elevated E2 levels before progesterone supplementation in FET cycles on pregnancy rates.
METHODOLOGY
The present study is a hospital-based retrospective analysis, conducted at Southend Fertility and IVF Centre, New Delhi. The aim of the present study is to study the impact of elevated E2 levels in FET cycles on pregnancy rates using six different protocols. As it was a retrospective analysis, no financial and ethical clearance required. The sample size was 242 patients who underwent FET of the cryopreserved embryos between September 2015 and April 2017.
Inclusion criteria
All patients who underwent FET of the cryopreserved embryos from previous IVF/ICSI cycles were included in this group. A detailed history and clinical examination were carried out and recorded on a predesigned proforma after screening for inclusion criteria. All the patients had routine investigations (as per proforma). All the patients had pelvic scan performed at day 2 of the cycle. Transvaginal scan was carried out and endometrial thickness was noted along with other parameters. Endometrial preparation was carried out with injectable/oral hormonal preparations using six different protocols. Endometrial preparation was carried out to increase the uterine receptivity before embryo transfer [Figure 1 and Table 1].
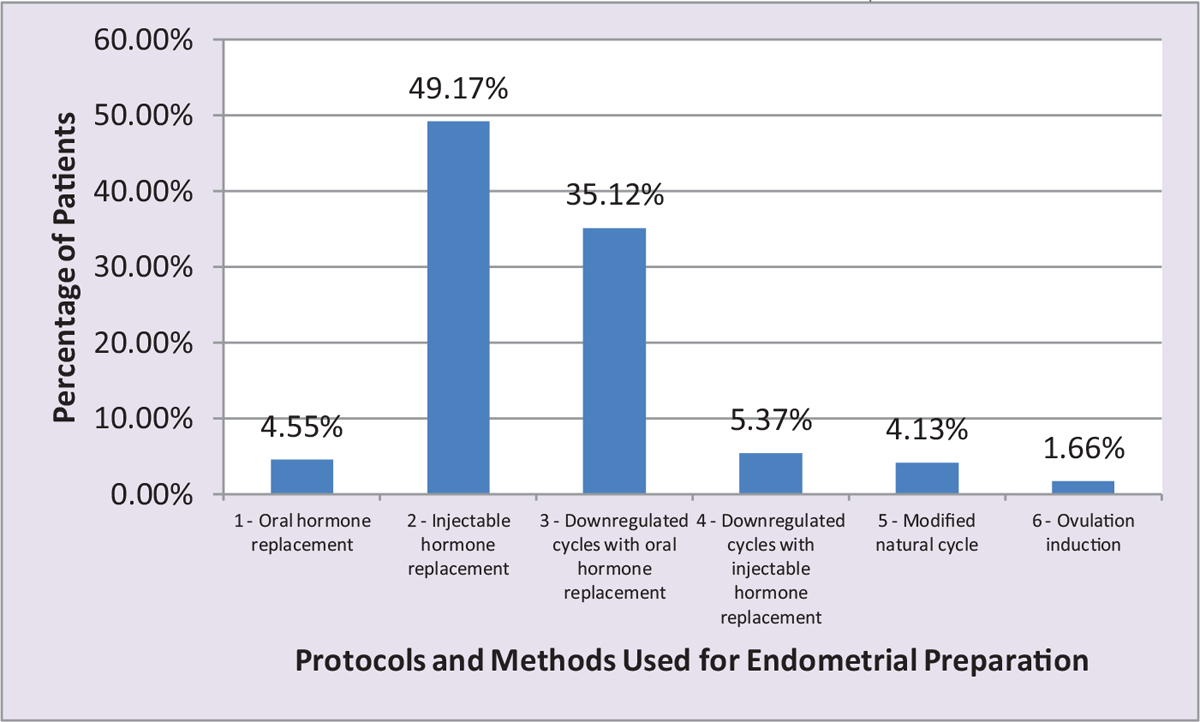
- Bar chart showing distribution of patients according to the number of patients in six different protocols in the study and the methods used for endometrial preparation.

PROTOCOLS
Protocol 1 (oral hormone replacement)
In oral hormone replacement, E2 valerate was started in early follicular phase, which is day 2/3 of the cycle following a step-up protocol. Having given 2 mg twice daily for 3 days, the dose was stepped to 2 mg thrice a day for 4 days. A review scan was performed to check the endometrial thickness on day 10 of the cycle and dose was adjusted accordingly. After an endometrial thickness of 7 mm was observed oral progesterone hormonal replacement was initiated.
Protocol 2 (injectable hormone replacement)
In this protocol, injectable E2 valerate 5 mg (Progynon; Zydus Cadila, India) intramuscular was given every third day till a desired endometrial thickness of 7 to 8 mm was achieved. This was again followed by the initiation of progesterone supplementation. Large number of patients were enrolled in this group as we were getting the desired results.
In patients with abnormal hormonal profile, endometrial preparation was carried out after pituitary downregulation. Patients were given oral contraceptive pills once a day for 15 days starting from day 2 of periods and subsequently on injection Lupride 0.5 mg sc daily once a day from day 13 of OCP. Downregulation was ensured if cycle day 2/3 hormone analysis revealed E2 < 60 pg/ml and P4 < 1 ng/ml, followed by either oral or injectable E2 supplementation.
Protocol 3 (downregulated cycles with oral hormone replacement)
Patients were given oral contraceptive pills once a day for 15 days starting from day 2 of periods and subsequently on injection Lupride 0.5 mg sc daily once a day from day 13 of OCP. E2 valerate was started in early follicular phase, which is day 2/3 of the cycle following a step-up protocol. Having given 2 mg twice daily for 3 days, the dose was stepped to 2 mg thrice a day for 4 days. A review scan was performed to check the endometrial thickness on day 10 of the cycle and dose was adjusted accordingly. After an endometrial thickness of 7 mm was observed oral progesterone hormonal replacement was initiated.
Protocol 4 (downregulated cycles with injectable hormone replacement)
Patients were given oral contraceptive pills once a day for 15 days starting from day 2 of periods and subsequently on injection Lupride 0.5 mg sc daily once a day from day 13 of OCP. Injectable E2 valerate 5 mg (Progynon; Zydus Cadila) intramuscular was given every third day till a desired endometrial thickness of 7 to 8 mm was achieved. This was again followed by the initiation of progesterone supplementation.
Protocol 5 (modified natural cycle)
Patients with ovulatory menstrual cycles and normal endocrine state were selected for modified natural cycle. After a day 2/3 check scan for uterus and ovaries, patient was followed up with repeat check scan for follicular monitoring and injection human chorionic gonadotropin (hCG) 10,000 IU was given intramuscularly. Follicular rupture was again checked with ultrasound and embryos transferred on day 3/5 accordingly.
Protocol 6 (ovulation induction)
Anovulatory patients were given Letrozole 2.5 mg once a day for 5 days, and a transvaginal sonogram was carried out on day 10 to measure endometrial thickness. After an endometrial thickness of more than 7 mm was observed on transvaginal sonogram, E2 levels were measured prior to progesterone supplementation with tablet Duphaston 10 mg twice daily (Dydrogesterone 10 mg; Abbott) and Susten (natural micronized progesterone; Sun Pharma) vaginal pessary 400 mg twice daily. After the embryo transfer, luteal support was given with injection Gestone 100 mg/day (Progesterone, 100 mg/2 ml; Ferring Pharma), and the impact of elevated E2 levels on pregnancy outcomes was evaluated in six different protocols. Pregnancy outcome was evaluated by using serum Beta hCG levels, 16 days after embryo transfer.
Statistical analysis
A descriptive analysis was carried out. Categorical variables were presented in number and percentage and continuous variables were presented as mean ± standard deviation. Comparison between different protocols was performed by using Mann–Whitney test and paired t test. All statistical analyses were performed with SPSS for Windows ver. 20 (IBM SPSS Statistics for Windows version 20).
Mean age of the patients in the study group was 34.35 ± 6.12 years, mean body mass index (BMI) was 25.75 ± 6.00 kg/m2, only 5.3% patients had BMI ≥30 and the rest of the patients had BMI <30.
Estrogen is an ovarian steroidal hormone, which is essential for the maintenance of pregnancy along with progesterone. E2 level was measured at day 2 or 3 of the cycle. Out of 242 patients in this study, maximum number of patients (205) had E2 levels in the range of 10 to 14 pg/ml (84.71%) and least in the range of 25 to 29 pg/ml. Mean E2 level was 11.83 ± 5.50 pg/ml. In the study by Hild-Petito et al.,[21] mean baseline (day 2 of the cycle) E2 levels in all the three study groups were 42 ± 12.4, 52 ± 15.5, and 50 ± 17.9, respectively. These values are little higher than the baseline E2 level in our study.
Total number of women enrolled in the present work was 242. Maximum patients, 119 (49.17%), were in Protocol 2 (injectable hormone replacement), followed by 85 (35.12%) in Protocol 3 (downregulated cycles with oral hormone replacement), and the least number of patients 4 (1.65%) were in Protocol 6 (ovulation induction).
The E2 levels were measured prior to progesterone supplementation and the impact of elevated E2 levels on pregnancy outcomes was evaluated in six different protocols. Mean of these elevated levels of E2 was measured for each protocol and a comparison was carried out between the patients getting pregnant and not getting pregnant in each protocol. Area under the curve was calculated and receiver operating characteristic (ROC) curves were plotted for each of the protocol. The results of the present work proved that elevated mean E2 levels had no significant impact on pregnancy rates in six different study protocols (oral and injectable) used for endometrial preparation [Figures 2-4].

- Receiver operating characteristic (ROC) curves for Protocols 1 and 2.

- Receiver operating characteristic (ROC) curve for Protocol 3.

- Receiver operating characteristic (ROC) curves for Protocols 5 and 6.
The findings of a similar study by Fritz et al.[34] showed that elevated mean E2 values in artificial frozen embryo replacement cycles had an adverse effect on live-birth/ongoing pregnancy rates. There is no defined optimum levels of E2 which is said to be related to optimum IVF-FET results. Wu et al.[35] evaluated the impact of high levels of E2 on the outcomes of IVF and concluded that there is no negative impact of elevated E2 levels on IVF outcome. The authors also concluded that may be there is a threshold highest E2 level above which pregnancy and implantation rates are lower, but this threshold is likely to be 5000 pg/ml and further randomized studies are required to define the optimal range of E2 level which is associated with best IVF-ET outcome.
RESULTS
Mean age of the patients in the study group was 34.35 ± 6.12 years. Maximum patients (n = 236) were below 40 years of age (97.53%). Twenty-five (10.34%) females were able to carry the pregnancy to a viable gestational age (Parity 1) and rest were nulliparous (89.66%). Maximum number of patients (n = 205) had E2 levels in the range of 10 to 14 pmol/l (84.71%) and least (n = 3) in the range of 25 to 29 pmol/l (1.23%). Mean E2 level was 11.83 ± 5.50 pg/ml progesterone (P4) levels were measured at day 2 or 3 of the cycle. Maximum number of patients (n = 236) had progesterone levels less than 1 nmol/l (97.52%) and six patients had baseline progesterone levels in the range of 1 to 3 nmol/l (2.48%). Mean progesterone level measured was 0.42 ± 0.52 nmol/l. The number of embryos transferred in the cycles varied from 1 to 4. Maximum number of embryos transferred was 3 in 133 patients (54.95%). In 87 patients (35.95%), 2 embryos were transferred, in 14 patients (5.78%), one embryo was transferred. Mean number of embryos transferred was 2.55 ± 0.26.
Maximum number of patients, 119 (49.17%), were in Protocol 2 (injectable hormone replacement), followed by 85 (35.12%) in Protocol 3 (downregulated cycles with oral hormone replacement), and the least number of patients 4 (1.65%) were in Protocol 6 (ovulation induction) [Tables 2 and 3].


DISCUSSION
This single-center retrospective analysis did not detect a correlation between the serum E2 level prior to the induction of the luteal phase and the subsequent live birth rates (LBR) in artificial FET cycles. Its conclusion is in line with the majority of formerly published work. Fritz et al.[34] included multiple artificial preparation protocols, adjusted the E2 supplementation dose in function of proliferative-phase serum E2 level measurements, and performed hormone analysis twice a week to calculate mean and peak values instead of focusing on one value at the time of luteal-phase induction. High serum E2 values have indeed been associated with poorer outcome by others as well.
The data showed once more that the natural cycle is adequately suppressed by early administered E2 supplementation and that, if serum P is used to detect escape ovulation, there seems to be no need for GnRH agonist cotreatment. In conclusion, no association between the mid-cycle serum E2 values and the subsequent LBR in artificial FET cycles was found. Therefore, the routine monitoring and use of these measurements to guide clinical decisions (e.g., medication step-up, cycle prolongation, or cancellation) are of questionable value.
The primary aim of this study was to evaluate the impact of elevated E2 levels in FET cycles on pregnancy rates using six different protocols, used for ovarian stimulation. This study is another step in the ongoing research in the field of ART. E2 levels were measured prior to progesterone supplementation and the impact of elevated E2 levels on pregnancy outcomes was evaluated in six different protocols. Mean of these elevated levels of E2 was measured for each protocol and a comparison was carried out between the patients getting pregnant and not getting pregnant in each protocol. Area under the curve was calculated and ROC curves were plotted for each of the protocol. The results of the present study prove that elevated E2 levels had no statistically significant impact on pregnancy rates in six different study protocols (oral and injectable) used for endometrial preparation.
The results of a similar study by Fritz et al.[34] showed that elevated average E2 levels in artificial autologous FET were adversely associated with live-birth /ongoing pregnancy rates.
There is no defined optimum range of E2 level which is associated with best IVF-FET results. Wu et al. evaluated the impact of high E2 levels on the outcomes of IVF and concluded that there is no adverse effect of elevated E2 levels on IVF outcome. The authors also concluded that may be there is a threshold peak E2 level above which pregnancy and implantation rates are decreased, but this threshold is likely to be 5000 pg/ml and further randomized studies are needed to define the optimum E2 range associated with best IVF-ET outcome.
In another recent study by Cerillo et al.,[36] impact of endometrial preparation protocols for FET on live-birth rates was analyzed. This was a prospective study, in which 530 patients (570 FET cycles) were randomly allocated to two study groups: Group 1 (n = 280 cycles), artificial cycle hormone replacement therapy (HRT); or group 2 (n = 290 cycles), natural cycle. Natural cycles were later divided into two groups: 169 patients scheduled with hCG and 121 with endogenous luteinizing hormone surge. This study suggests that endometrial preparation carried out in either group by the above-mentioned protocols resulted in similar implantation rates, clinical and ongoing pregnancy rates, and live-birth rates.
SUMMARY AND CONCLUSION
In light of current literature, there is no defined optimum range of E2 level which is associated with best IVF-FET results and on the basis of the results in the present study, we conclude that elevated E2 levels had no statistically significant impact on pregnancy rates in six different study protocols, using different routes of hormonal administration (oral and injectable) used for endometrial preparation.
As per our knowledge and after search in the PubMed, the present study is the first of its kind wherein we compared the impact of elevated E2 levels in FET cycles on pregnancy rates and the endometrial preparation was carried out by six different protocols using different routes of hormonal administration, whether injectable or oral.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707-9.
- [Google Scholar]
- Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod. 2006;21:2368-74.
- [Google Scholar]
- Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94:1320-7.
- [Google Scholar]
- 29th Annual Meeting of the European Society of Human Reporduction and Embryology. In: Focus on Reproduction. London, UK: ESHRE; 2012.
- [Google Scholar]
- The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocrine Rev. 2006;27:170-207.
- [Google Scholar]
- Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768-74.
- [Google Scholar]
- Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344-8.
- [Google Scholar]
- Evidence of impaired endometrial receptivity after ovarian stimulation for in-vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril. 2011;96:516-8.
- [Google Scholar]
- Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84-90.
- [Google Scholar]
- Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86:862-6.
- [Google Scholar]
- Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98:368-77.
- [Google Scholar]
- High ongoing pregnancy rates after deferred transfer through bipronuclear oocyte cryopreservation and post-thaw extended culture. Fertil Steril. 2009;92:1594-9.
- [Google Scholar]
- Cryopreserved zygotes and embryos and endocrinologic factors in the replacement cycle. Fertil Steril. 1988;50:61-7.
- [Google Scholar]
- Controlled preparation of the endometrium with exogenous steroids for the transfer of frozen-thawed pre-embryos in patients with anovulatory or irregular cycles. Hum Reprod. 1991;6:443-5.
- [Google Scholar]
- Transfer of cryopreserved-thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin-releasing hormone agonist and exogenous estradiol and progesterone (GEEP) Fertil Steril. 1989;52:609-16.
- [Google Scholar]
- Estrogen level monitoring in artificial frozen-thawed embryo transfer cycles using step-up regime without pituitary suppression: is it necessary? J Experi Clin Assist Reprod. 2008;5:4.
- [Google Scholar]
- Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334-40.
- [Google Scholar]
- Biochemical and immunohistochemical analyses of estrogen and progesterone receptors in the rhesus monkey uterus during the proliferative and secretory phases of artificial menstrual cycles. Fertil Steril. 1990;53:913-20.
- [Google Scholar]
- Histology of the human endometrium: from birth to senescence. Ann NY Acad Sci. 1991;622:6-27.
- [Google Scholar]
- Immunocytochemical localization of estrogen and progestin receptors in the baboon (Papio anubis) uterus during implantation and pregnancy. Endocrinology. 1992;130:2343-53.
- [Google Scholar]
- Oestrogen and progesterone receptors in endometriotic tissue and endometrium: comparison of different cycle phases and ages. Hum Reprod. 1993;8:2211.
- [Google Scholar]
- Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925-30.
- [Google Scholar]
- Immunohistochemical analysis of estrogen receptor α, estrogen receptor β and progesterone receptor in normal human endometrium. Acta Histochem. 2004;106:245-52.
- [Google Scholar]
- The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170-207.
- [Google Scholar]
- Endometrial thickness: a predictor of implantation in ovum recipients? Hum Reprod. 1994;9:363-5.
- [Google Scholar]
- Long oestradiol replacement in an oocyte donation programme. Hum Reprod. 1995;10:1387-91.
- [Google Scholar]
- Effect of duration of estradiol replacement on the outcome of oocyte donation. J Assist Reprod Genet. 2001;18:187-92.
- [Google Scholar]
- An insight into early reproductive processes through the in vivo model of ovum donation. J Clin Endocrinol Metab. 1991;72:408-14.
- [Google Scholar]
- Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril. 2002;77:956-60.
- [Google Scholar]
- Increasing uterine receptivity by decreasing estradiol levels during the preimplantationperiod in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil Steril. 1998;70:234-9.
- [Google Scholar]
- Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86:862-6.
- [Google Scholar]
- Current status of human oocyteand embryo cryopreservation. Curr Opin Obstet Gynecol. 2011;23:245-50.
- [Google Scholar]
- Elevated estradiol (E2) levels in artificial autologous frozen embryo transfer (FET) cycles negatively impact live birth (lb) and ongoing pregnancy (OP) rates. Fertil Steril. 2017;107:e43.
- [Google Scholar]
- High serum estradiol levels are not detrimental to in vitro fertilization outcome. Taiwan J Obstet Gynecol. 2007;46:54-9.
- [Google Scholar]
- Impact of endometrial preparation protocols for frozen embryo transfer on live birth rates. Rambam Maimonides Med J. 2017;8:e0020.
- [Google Scholar]







