Translate this page into:
Booster dose of human chorionic gonadotrophin for unruptured follicle in non-IVF/ICSI cycles: a randomized controlled study
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To assess the effect of booster dose of human chorionic gonadotropin (HCG) on the unruptured follicles in non-in vitro fertilization/intracytoplasmic sperm injection cycles.
Setting and design:
This randomized controlled multicenter study was conducted at Tanta and Zagazig University hospitals in the period from August 1, 2018 to January 1, 2021.
Patients and methods:
Enrolled patients (n = 160) who had unruptured mature Graafian follicle 48 hours after intramuscular (IM) 10,000 IU HCG dose. Patients were allocated randomly into intervention group and control group. In the intervention group, another booster dose (10,000 IU of HCG) was given IM, whereas in the control group, 1 mL normal saline was given IM.
Study outcomes:
Ovulation and pregnancy rates.
Results:
The number of cases who had follicular rupture was similar to those in the control group. The incidence of luteinized unruptured follicle (LUF) was nearly similar in both groups. No significant differences were noticed regarding pregnancy rates in both groups.
Conclusion:
Booster dose of HCG for unruptured follicles has no benefit in ovulation triggering nor in the prevention of LUF syndrome. There was no significant difference in pregnancy rates in both groups.
Keywords
Booster dose
gonadotropins
HCG
LUF
ovulation rates
pregnancy rates
INTRODUCTION
Ovulation is associated with significant hormonal changes, and the process is primarily under the control of luteinizing hormone. This is the hormone that rises in the mid-cycle surge about 36 hours before ovulation and is the basis for home ovulation predictor kits. Ovulation is a process that occurs over a span of time − it is not a full blown explosion as many people think.[1]
During ovulation, cellular chemicals called prostaglandins and proteolytic enzymes weaken the wall of the follicle containing the egg, resulting in a rupture through which the egg is released. The egg then enters the fallopian tube where it awaits fertilization with sperm.[2]
Most tests that are used to confirm ovulation document only the hormonal changes. Serial ultrasound studies can be used to follow the growth of the follicle and its collapse following ovulation. In a small percentage of normal women, the dominant follicle will occasionally undergo the luteinization process without rupture following the mid-cycle surge.[3] As a result of the increased progesterone secretion, the uterine lining develops its normal characteristic changes following ovulation, but no oocyte is released and conception cannot occur. Luteinized unruptured follicle (LUF) is a form of anovulation in which ovulation does not occur successfully. This is a rare condition and can prevent conception in many women; this phenomenon is called the LUF.[4]
The true incidence of this problem in both the fertile and infertile population is unknown but is thought to be quite low. The majority of the studies on this topic were published 25 to 35 years ago but these studies were of very poor quality, often conducted with very few patients or without a comparison group. LUFs sometimes occur more frequently in women taking fertility supplements or prescriptions and who have had pelvic inflammatory disease, polycystic ovarian syndrome (PCOS), and endometriosis.[5]
Many studies attempting to describe LUF instead described what we now recognize as ovulation dysfunction and subtle abnormal hormonal parameters of ovulation. The normal corpus luteum can sometimes look like a preovulatory follicle during its initial stages, so capturing the follicular collapse was sometimes difficult to discern, especially with the equipment available at that time. This means that the prevalence of the problem may have been overestimated. Therefore, the true magnitude and scope of LUF remains a mystery; some studies found association between nonsteroidal anti-inflammatory drug (NSAID) and LUF syndrome.[6]
Fortunately, this problem is easily resolved by ovulation induction medication and by “triggering” ovulation via an appropriate dose of injectable human chorionic gonadotropin (HCG) or gonadotropin-releasing hormone agonist (such as Lupron).[7]
The LUF has been demonstrated in both spontaneous and stimulated cycles. As the possibility of LUF is still present in ordinary stimulated cycles, the role of booster dose of trigger by HCG is questionable and whether increases ovulation and pregnancy rates or not. In addition, the role of booster dose of trigger by HCG in reducing incidence of LUF syndrome is still unclear. For these issues, this study was conducted.[8]
PATIENTS AND METHODS
Study design and settings
This study is a double-blinded, randomized controlled multicenter study conducted at Tanta and Zagazig University hospitals in the period from August 1, 2018 to January 1, 2021.
Patients
A total of 160 infertile women were initially enrolled on outpatient basis in the fertility clinic of Tanta and Zagazig University hospitals. Eligible patients were selected according to inclusion and exclusion criteria. The inclusion criteria were (i) age 20 to 35 years, (ii) anovulatory infertility, (iii) ovulation induction by clomiphene citrate, and (iv) normal other investigations of infertility. The exclusion criteria are (i) patients with clomiphene citrate resistance, (ii) hyperprolactinemia, (iii) endometriosis or ovarian cyst detected by ultrasound on days 3 to 5 of the menstrual cycle, and (iv) patients on NSAID therapy.
Sample size calculation
At 5% of type 1 error and precision of 5%, sample size was calculated in Epi Info version 7 (CDC, Atlanta, USA) program with the result of 94 patients as a sample size.
Intervention
Patients were given clomiphene citrate (Tecnovula; TECHNO Company, Technovula®, Techno pharmaceutical, Egypt) in third day of menstruation for 5 days in a dose ranging from 50 to 150 mg/daily. Dose was increased by 50 mg per cycle according to patients’ response.
Transvaginal ultrasound (3.5 mHz) was performed on day 8 of the menstrual cycle and then every other day. The diameters of follicle(s) were measured in the transverse and longitudinal planes, until mature follicles emerged with diameter of 18 to 22 mm.
Ovulation was triggered with HCG 10,000 IU (Choriomon®, IBSA, Switzerland) administered intramuscularly. Patients were followed up after 48 hours to check occurrence of ovulation by disappearance of leading follicle or its shrinkage to its half size and the presence of free fluid in pelvis. Patients who had at least one follicle ruptured are excluded from the study.
Patients who had no follicular rupture after HCG triggering with persistence of leading follicle(s) were included in the current study. Patients were further allocated into either study group or control group. The study group received another booster dose of HCG 10,000 IU (Choriomon®, IBSA, Switzerland) intramuscularly, whereas the control group received placebo in the form of 1 mL of normal saline 0.9% intramuscularly. Another follow-up ultrasound 48 hours after second triggering was carried out to check for ovulation and rupture of follicle(s).
Patients were instructed to undergo sexual intercourse every other day starting the day before booster dose of HCG injection until ovulation window ends. Serum progesterone was measured 1 week after booster dose administration. All patients underwent serum pregnancy test 14 days after booster dose of HCG administration.
Randomization and allocation
Patients were randomized by electronic computerized program using codes for allocation group. The codes are put in a closed envelope. The allocation of patients who open envelope was not changed. Allocation was equal 1:1.
Methods
Demographic data of all patients were registered. Basal hormonal profile, dose of clomiphene citrate, duration of stimulation, number of follicle yielded, number of follicles ruptured after booster dose, ovulation, and pregnancy rates were recorded.
Outcomes of study
The primary outcome was ovulation rate, whereas the secondary outcome was pregnancy rate.
Ethical approval and study registration
Ethical committee of Tanta and Zagazig University approved this study on August 18, 2018 and the study code is 32518/08/18. The study was registered on Clinicaltrials.gov with the unique ID: NCT03580031 and is available on the following link: https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S000847W&selectaction=Edit&uid=U000404W&ts=2&cx=-pat8jz
Statistical methods
The statistical analysis was carried out using SPSS version 18 (IBM® SPSS, version 18, NY, USA). Descriptive statistical were tests used in the current study. These tests were mean, standard deviation, Chi-squared, and P-value. Significance is considered when P-value is ≤0.05.
RESULTS
One hundred and sixty infertile women were included in the current study. After mature Graafian follicles were reached, triggering was given. Patients were assessed by ultrasound to check ovulation. Patients with ovulation signs (n = 90) were excluded. Patients presented with no follicular rupture (n = 70) were further allocated into either study group (n = 35) or control group (n = 35). The flow chart of cases all through the study is shown in Figure 1.
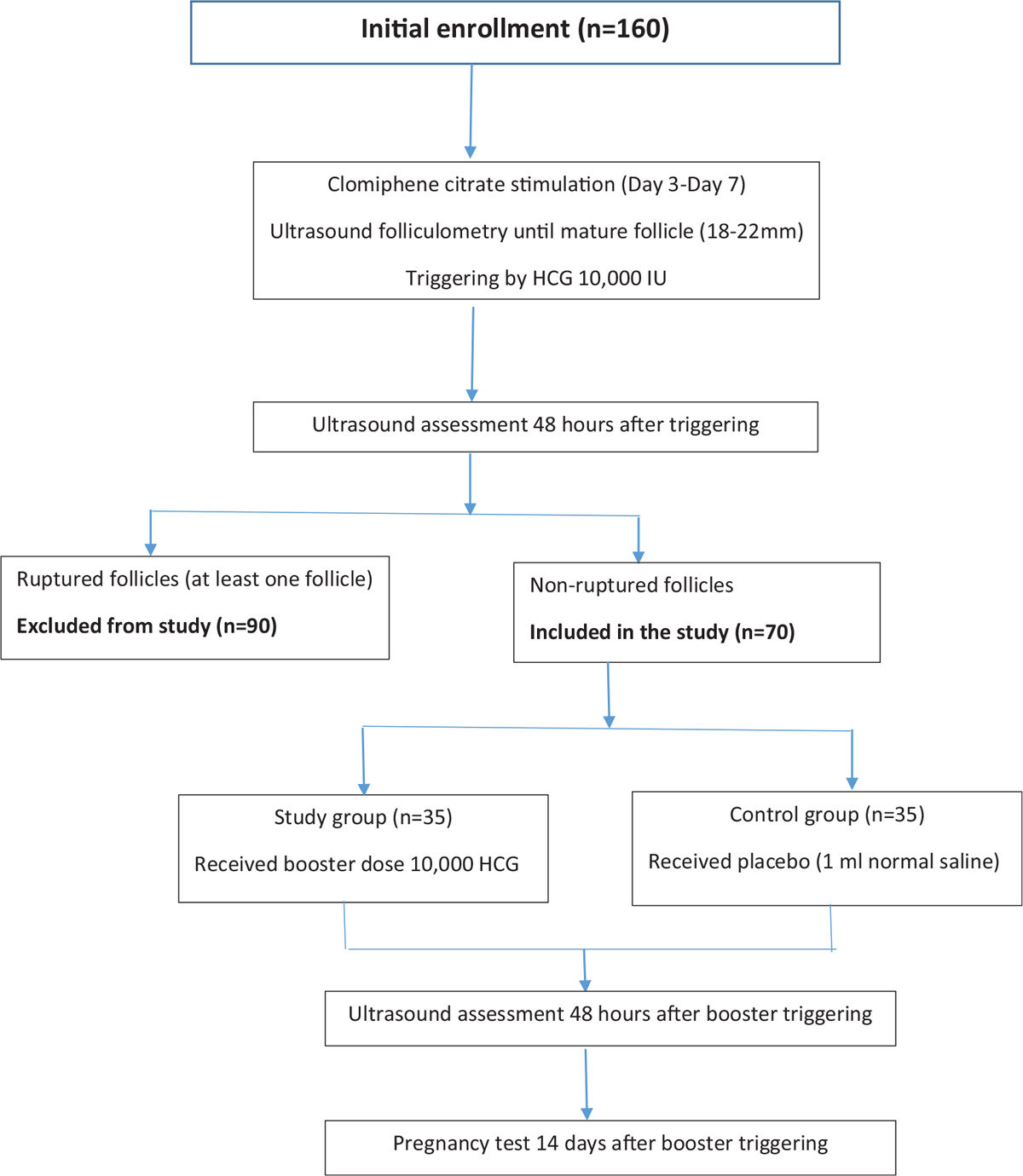
- Flow chart of enrolled patients and their allocation all through the study.
Demographic data and basal hormonal profile of enrolled patients (n = 160) are presented in Table 1. The mean age was 27.05 ± 4.23 years. The mean gravidity was 0.430 ± 0.847, whereas the mean parity was 0.738 ± 0.107. The great majority of infertile patients were suffering primary infertility 101 (63.1%). The average duration of infertility was 1.95 ± 0.75 years. Cesarean section was the prevalent mode of delivery 43 (26.88%) in patients with secondary infertility. The hormonal profile and ovarian volume were normal in all infertile patients.

The mean number of mature follicles was 2.033 ± 0.296. After the first dose of HCG, the ovulation occurred in 90 (56.25%) of patients who were excluded from the study and were followed up as usual for occurrence of pregnancy. The patients who had no ovulation 70 (43.75%) were included in the study and given the treatment according to their allocation. The mean endometrial thickness was 10.6 ± 1.44 mm. The data of first dose HCG are presented in Table 2. Patients with persistent nonruptured follicles are shown in Figure 2.


- Unruptured follicle after first booster dose of human chorionic gonadotropin.
The data of patients in study and control groups are presented in Table 3. After booster dose of HCG, only 10 (28.57%) had ovulation in study group, whereas in control group after placebo, ovulation occurred in 8 (22.85%) with nonsignificant difference between both groups (P = 0.586). Patients with nonruptured follicles after booster dose HCG are shown in Figure 3. The mean mid-luteal progesterone level was 15.17 ± 3.40 ng/mL in study group and was 14.10 ± 3.63 ng/mL in control group with nonsignificant difference between both groups (P = 0.207). Regarding pregnancy rates after booster dose, two (5.71%) patients got pregnant in study group and three (8.57%) patients in control group with nonsignificant difference between both groups (P = 0.644).


- Unruptured follicle after second booster dose of human chorionic gonadotropin.
DISCUSSION
The LUF occurs due to the absence of oocyte expulsion from primary follicle or failure of ovulatory follicle to rupture. The incidence of LUF is about 4.9% to 10% in normal fertile women, but a higher incidence has been reported in infertile women after 2 days of HCG in 68% of spontaneous cycles and in 81% (95% confidence interval 76–86) in stimulated cycles. Ovulation was evident by follicular rupture.[9,10]
In the current study, the incidence of LUF syndrome was 43% in which signs of ovulation of ultrasound were not noticed. Thirty-five patients were given booster dose of HCG, whereas 35 patients were given saline, there was ovulation in 10 patients in intervention group and 8 patients in control group. Ovulation was documented by ultrasound criteria and mid-luteal serum progesterone. Pregnancy occurred in two patients in intervention group and three patients in control group.
Qublan et al. showed that incidence of LUF was (12%) among 25 intrauterine insemination (IUI)-stimulated cycles, Devroey et al. reported that incidence of LUF was 11.8%. Hamilton et al. cleared that incidence of LUF was 6.7% among 270 infertile women underwent 600 treatment cycles.[11,12,13]
Luciano et al. showed that among 50 infertile women, 6% of patients were diagnosed LUF syndrome. Bateman et al. reported same results.[2] Luciano et al. concluded that 20% of their patients were treated with clomiphene citrate had LUF syndrome.[9,14] Bateman et al., Qublan et al., and Ghanem et al. reported that incidence and recurrence rates of LUF syndrome are increased significantly in consecutive cycles stimulated with clomiphene citrate.[2,11,14] These finding are in agreement with our study, which may indicate that clomiphene citrate may have role in etiology of LUF syndrome. Koninckx and Brosens showed that LUF occurs statistically more frequently in women with unexplained infertility than in a control.[15] This was consistent with our study which included unexplained infertile patients. The higher incidence of LUF in our study may be due clomiphene citrate as some studies denoted that letrozole had lower incidence of LUF than CC when given for induction of ovulation in IUI cycles.[16]
Regarding the role of booster dose of HCG, Deepika et al. showed that booster dose of GnRHa 12 hours following the first dose in PCOS undergoing in vitro fertilization with antagonist provides a better cycle outcome than a single dose in terms of maturity of oocytes, higher number of blastocysts, and a trend toward improvement in clinical pregnancy.[17] de Mola showed that booster dose of HCG do not solve problem of LUF.[18] Our study showed that booster dose of HCG for unruptured follicles has no benefit in ovulation triggering.
Another study was conducted by Shibata et al. to test the value of granulocyte colony-stimulating factor (G-CSF) in combination with the ovulation induction in reducing the incidence of LUF syndrome. Their study concluded that additional use of G-CSF significantly prevented LUF syndrome during ovulation induction without serious adverse reactions.[19] Our study showed that booster dose of HCG for unruptured follicles has no benefit in ovulation triggering.
In the current study, pregnancy occurred in two patients in intervention group and three patients in control group. The normal corpus luteum can sometimes look like a preovulatory follicle during its initial stages, so detecting the follicular collapse was sometimes difficult. This may explain pregnancy in these patients.
The strength points of the current study were the randomized nature of the study, addressing a common problem in unexplained infertility, and this may help to solve the problem of LUF syndrome. In addition, the effect of booster dose of HCG on LUF was studied. One of the limitations of this study is the small sample size.
CONCLUSION
Booster dose of HCG for unruptured follicles has no benefit in ovulation triggering. There was no significant difference in pregnancy rates in both study and control groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Luteinized unruptured follicle syndrome: a subtle cause of infertility. Fertil Steril. 1978;29:270-4.
- [Google Scholar]
- Pelvic sonography can predict ovum release in gonadotrophin-treated patients as determined by pregnancy rate. Hum Reprod. 1990;5:234-6.
- [Google Scholar]
- Luteinized unruptured follicle: morphology, endocrine function and blood flow changes during the menstrual cycle. Hum Reprod. 1995;10:44-9.
- [Google Scholar]
- Increased incidence and recurrence of recent corpus luteum without ovulation stigma (luteinized ruptured follicle syndrome) in baboons with endometriosis. J Soc Gynecol Invest. 1996;3:140-4.
- [Google Scholar]
- Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod. 2001;16:1323-8.
- [Google Scholar]
- Gonadotropin-releasing hormone agonist versus human chorionic gonadotropin for ovulation triggering in letrozole stimulated cycles. Middle East Fertil Soc J. 2018;23:303-9.
- [Google Scholar]
- The luteinized unruptured follicle other ovulatory dysfunctions. Fertil Steril. 1988;50:839-50.
- [Google Scholar]
- Temporal relationship and reliability of the clinical, hormonal, and ultrasonographic indices of ovulation in infertile women. Obstet Gynecol. 1990;75:412-6.
- [Google Scholar]
- The temporal relation between the urine LH surge and sonographic evidence of ovulation: determinants and clinical significance. Obstet Gynecol. 1994;83:184-8.
- [Google Scholar]
- Luteinized unruptured follicle syndrome: incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum Reprod. 2006;21:2110-3.
- [Google Scholar]
- Origin of low peritoneal fluid steroid concentrations in the luteinized unruptured follicle syndrome. In: Thompson W, Harrison RF, Bonnar J, eds. Ovulation and Its Disorders. Dordrecht: Springer; 1984. p. :79-83.
- [Google Scholar]
- Follicle growth curves and hormonal patterns in patients with the luteinized unruptured follicle syndrome. Fertil Steril. 1985;43:541-8.
- [Google Scholar]
- Effect of stimulation protocol on the risk of luteinized unruptured follicle (LUF) in polycystic ovarian syndrome (PCOS): does LUF affect luteal phase profile? Fertil Steril. 2009;92:S99.
- [Google Scholar]
- Clinical significance of the luteinized unruptured follicle syndrome as a cause of infertility. Eur J Obstet Gynecol and Reprod Biol. 1982;13:355-68.
- [Google Scholar]
- Effects of letrozole-HMG and clomiphene-HMG on incidence of luteinized unruptured follicle syndrome in infertile women undergoing induction ovulation and intrauterine insemination: a randomised trial. Glob J Health Sci. 2016;8:244-52.
- [Google Scholar]
- Repeat dose of gonadotropin-releasing hormone agonist trigger in polycystic ovarian syndrome undergoing in vitro fertilization cycles provides a better cycle outcome − a proof-of-concept study. J Hum Reprod Sci. 2017;10:271-80.
- [Google Scholar]
- Principles and practice of assisted reproductive technology. Fertil Steril. 2014;102:610.
- [Google Scholar]
- Granulocyte colony-stimulating factor as a potential inducer of ovulation in infertile women with luteinized unruptured follicle syndrome. Transl Res. 2016;171:63-70.
- [Google Scholar]







